Abstract
A cyclam (1,4,8,11-tetraazacyclotetradecane)-based macrocycle bearing two benzyl and two 2-hydroxy-3,5-di-tert-butylbenzyl pendent arms was synthesized and characterized using spectroscopic techniques and single crystal X-ray diffraction. The macrocycle crystallizes in the triclinic space group P-1, with the asymmetric unit containing one-half of the molecule. The structure is stabilized by hydrogen-bonding which exists between the phenolic protons and the nitrogen atoms of the macrocyclic ring. The presence of this hydrogen bonding is observed in the 1H-NMR due to the deshielded nature of the phenolic OH peak (δ 9.99). Cyclic voltammetry of the ligand revealed a single quasi-reversible peak at −0.58 V (Epc = −0.48 V and Epa = −0.68 V), which is due to the electrochemical oxidation of the phenol to the phenoxyl radical.
1. Introduction
The N-functionalisation of cyclam (1,4,8,11-tetraazacyclotetradecane) to produce macrocyclic ligands continues to be of significant interest due to the very stable complexes that result when complexed to metal ions. Subsequently, complexes of these ligands have potential to be utilised for medical imaging and therapy, such as anti-HIV drugs [1,2,3,4], magnetic resonance imaging (MRI) contrast agents [5] and positron emission tomography (PET) theranostic compounds [6,7,8,9]. The strong affinity that these ligands have for important metal ions, such as copper(II), has seen them becoming increasingly used in the formation of sensors for either environmental analysis or cellular studies [10,11,12].
The choice of pendent arm with which to N-functionalise cyclam is of critical importance for aiding the resulting metal complex achieve its desired function. One of the main roles of a pendent arm is to increase the available number of coordination atoms available to the central metal ion. Crucially, the ligand should meet the required coordination demand of the metal ion in question in order to maximise stability. Another role of the pendent arm may be to provide an attachment point for a biomolecule to direct the resulting complex towards a certain bio-marker [13].
Phenolate pendent arms appended to macrocycles have been the focus of a number of groups. Maria and co-workers used bulky phenolate pendent arms to provide steric protection to chelated rare-earth and actinide metal ions [14,15]. 2,4-dimethylphenol was used as a pendent arm to decorate the periphery of 1,4,7-triazacyclonane to increase the hydrophobicity of the resulting gallium(III) complex in order to study its potential in radiotherapy [16]. The prospect of generating stabilised phenoxyl radical species has also led to macrocyclic complexes bearing phenolate pendent arms being studied for this purpose and to elucidate the effect that the chelated metal ions have on the redox potential [17,18]. The fact that copper-containing proteins such as cyctochrome c oxidase, photosystem II and galactose oxidase utilise tyrosyl radicals also makes these systems attractive to study [19,20].
In this paper, the synthesis, characterisation and X-ray structure of a cyclam macrocycle bearing two benzyl and two phenol pendent arms is detailed. The redox properties of the phenol pendent arm are probed by cyclic-voltammetry.
2. Results and Discussion
2.1. Synthesis and Characterisation of the Title Compound
The synthetic strategy for the synthesis of the title compound 4, is shown in Scheme 1. In order to control the nucleophilicity of the nitrogen atoms of cyclam, methylene bridges were introduced to rigidify the macrocyclic backbone, to give compound 1 [21]. The use of formaldehyde in this instance was preferred over glyoxal to yield the more reactive diammonium salt. Consequently, rigidifying the backbone resulted in only two of the four nitrogen atoms possessing exo lone pairs for nucleophilic attack of benzyl bromide. Thus, the isolation of compound 2 was facile, and was produced in an almost quantitative yield. Subsequent removal of the methylene bridges in basic media gave compound 3, analytical data for which matched existing literature data [21,22]. Subsequent N-alkylation with 2-hydroxy-3,5-di-tert-butylbenzyl was facilitated via an established Mannich procedure [14] by using formaldehyde and 2,4-di-tert-butylphenol to give compound 4 in 70% yield.
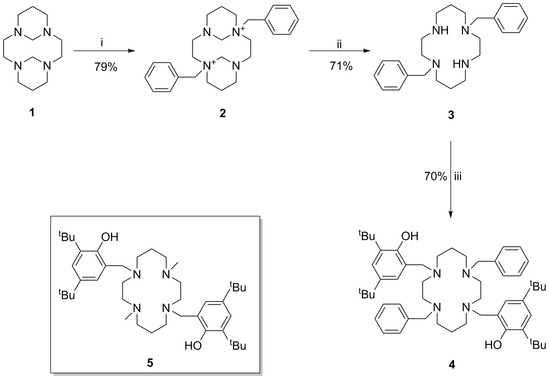
Scheme 1.
Synthetic scheme for the synthesis of 4. Brief conditions: (i) Benzyl bromide (2.1 eq); (ii) 3M NaOH, 3 hr; (iii) CH2O, 2,4-di-tert-butylphenol. The insert shows compound 5, the analogous methyl derivative of compound 4, which has been synthesised by Maria et al. [14].
2.1.1. NMR Analysis
Compound 4 possesses a downfield broad singlet at δ 9.99 in the 1H-NMR, which is representative of a hydrogen bonded phenolic OH. A similar chemical shift is reported for an analogous compound which exhibits H-bonding in the X-ray structure in which the benzyl moieties are exchanged for methyl groups (compound 5 in Scheme 1) [14]. The two proton resonances of the 2,4-di-tert-butylphenol pendent arms are observed at δ 7.17 and 6.69 and possess a common J-coupling of 2.4 Hz. The benzylic protons are also readily identifiable. However, the macrocyclic protons are more difficult to assign due to the similar chemical environments in which they exist.
2.1.2. Cyclic Voltammetry Analysis
The title compound possesses phenol pendent arms that are redox active. A single quasi-reversible peak is observed at −0.58 V (Epc = −0.48 V and Epa = −0.68 V) in the cyclic voltammogram over the scan rates 50 mV s−1 to 250 mV s−1 (Figure 1). In solution, the phenol undergoes an electrochemical oxidation process to generate the phenoxyl radical under the conditions employed in cyclic voltammetry, prior to being reduced to the phenolate anion. This is reflected in the variance of Ia/Io over the scan rates presented. This value compares well with a Ga(III) and Fe(III) triaazacyclononane based macrocyclic system which possesses a single 2,4-di-tert-butylphenolate pendent arm; redox potentials for the phenolate pendent arm are observed for these complexes at 0.63 and 0.73 V, respectively, in the respective cyclic voltammograms [18].
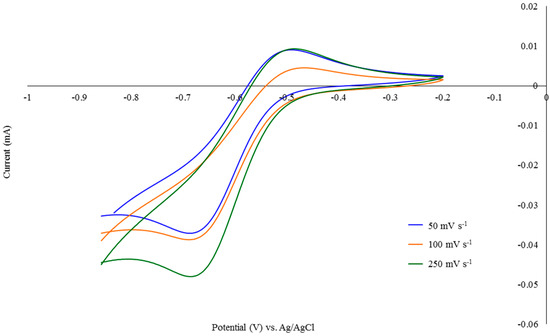
Figure 1.
Cyclic voltammagrams of compound 4 in CH3CN (0.1 M tetrabutylammonium perchlorate (TBAP)) recorded at scan rates of 50, 100 and 250 mV s−1 at a glassy carbon electrode at 20 °C.
2.1.3. X-ray Diffraction Analysis
Single crystals of compound 4 suitable for X-ray diffraction were grown from a concentrated chloroform solution. Compound 4 crystallizes in the triclinic space group P-1. The asymmetric unit contains one-half of the molecule with the macrocycle centred on a crystallographic inversion centre (−x, −y, −z). An ORTEP diagram is shown in Figure 2a.
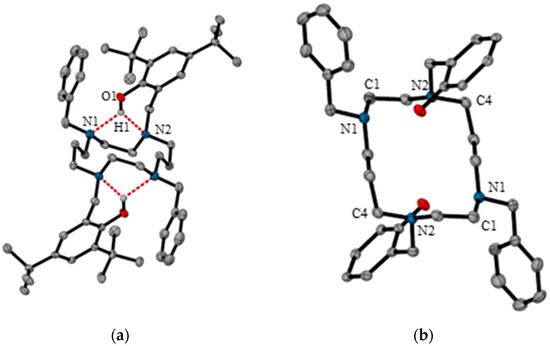
Figure 2.
(a) Molecular structure of compound 4 with all hydrogens removed for clarity (with the exception of H1). Thermal ellipsoids are drawn at 50% probability level. Hydrogen-bonding is denoted by dashed bonds; (b) Conformation of the cyclam ring in compound 4 (hydrogens and tert-butyl groups omitted for clarity). C1 and C4 are located at the corners of the rectangle.
The 14-membered macrocycle adopts a rectangular conformation, which according to Dale’s conventions is (3,4,3,4)-B [23,24]. In this conformation, symmetry related carbon atoms (C1 and C4) are located in the corners of the rectangle (see Figure 2b). The torsion angle sequence of the macrocyclic ring, τ1–τ7, is −167.8, 172.8, −66.7, −75.3, 173.1, −42.6, −67.0. In this sequence, τ1 is C1-N1-C2-C3 and τ7 is C5-C1-N1-C2. These values differ only slightly compared to an analogous macrocycle in which the benzyl pendent arms are replaced by methyl groups (compound 5) [14]. The configuration of the four nitrogen atoms of 4 correspond to a type IV cyclam, which, according to Bosnich’s nomenclature, has the chirality R,S,S,R [25].
The phenolic hydrogen was located in the difference map. The O-H bond length was fixed to 0.95 Å, and the thermal parameter of the hydrogen atom was set 1.2 times larger than the parent O-atom. Upon analysis, the phenolic hydrogen exhibited intramolecular hydrogen-bonding to the two nitrogen atoms located in the nearby vicinity. The distance between the phenolic hydrogen and the nitrogen atoms of the macrocyclic ring are 2.28 Å and 2.08 Å for N(1)-H(1)O(1) and N(2)-H(1)O(1) respectively (Table 1 contains the hydrogen-bond geometry data). These lengths are slightly longer than those observed in compound 5 (H···A = 2.251 and 1.990 Å) [14], although the phenolic hydrogen in this structure was geometrically fixed during refinement. However, the hydrogen-bond lengths of compound 4 are either commensurate or shorter than those of a further analogous compound which has the same structure as compound 5 except that the tert-butyl groups have been replaced for methyl groups (H···A = 2.412 and 2.070 Å) [26].

Table 1.
Hydrogen bond geometry (Å, °).
3. Materials and Methods
3.1. General Methods and Physical Measurements
All NMR spectra were acquired in the solvent indicated with chemical shifts quoted as parts per million values (ppm) and coupling constants (J) quoted in hertz (Hz). NMR spectra were recorded using a JEOL JNM-LA400 FT NMR spectrometer (Tokyo, Japan) at a frequency of 400 MHz for 1H spectra and 100 MHz for 13C spectra. Liquid chromatography mass spectrometry was performed using an Agilent 1260 Infinity LC system (Santa Clara, CA, USA). The FT-IR spectra were collected using a Thermo Fisher Scientific Nicolet 360 system (Waltham, MA, USA).
Cyclic voltammetry was performed using a standard three-electrode configuration with glassy carbon working electrode (0.3 mm diameter disk), silver counter electrode and an Ag/AgCl reference. All measurements were made in MeCN solution of 0.1 mol dm−3 [TBAP], unless otherwise stated, over the scan rates of 0.05 Vs−1 to 0.25 Vs−1.
3.2. Synthesis of 1,8-Bis(2-hydroxy-3,5-di-tert-butylbenzyl)-4,11-dibenzyl-1,4,8,11-tetraazacyclotetradecane
Chemicals were purchased from either CheMatech (cyclam) or Sigma-Aldrich and used as received. Dry acetonitrile (99.5%) was purchased from Acros Organics. Compounds 1–3 were synthesised according to established literature procedures [21].
Compound 3 (0.02 g, 0.053 mmol) was dissolved in methanol (20 mL) and a 37% of formaldehyde solution (0.015 mL) was added. The mixture was refluxed for 2 h under argon and after this period 2,4-di-tert-butylphenol (0.021 g, 0.10 mmol) in methanol (10 mL) was added. The reaction was heated to reflux and left overnight under an argon atmosphere. The white solid formed was filtrated, washed with methanol and dried under vacuum (0.03 g, 70%); 1H-NMR (CDCl3, 400 MHz) δ 9.99 (br s, 1H, Ar-OH), 7.37–7.36 (m, 2H, benzyl H), 7.17 (d, 1H, J = 2.4 Hz, Phen H), 7.07–7.05 (m, 3H, benzyl H), 6.69 (d, 1H, J = 2.4 Hz, Phen H), 4.25–4.16 (m, 2H), 3.99–3.22 (m, 4H), 2.89–1.94 (m, 6H), 1.48 (s, 9H, tBu), 1.27 (s, 9H, tBu), 0.92–0.87 (m, 2H); 13C-NMR (CDCl3) δ 153.4, 139.6, 139.3, 134.9, 129.8, 129.6, 127.9, 126.9, 124.4, 122.9 (Carom), 59.5, 56.1, 51.7, 50.3, 50.2, 48.6 (CH2), 35.0, 34.1 (C(tBu)), 31.8, 29.8 (Me(tBu)), 24.5 (CH2CH2CH2); HRMS (ESI-MS) expected for C54H81N4O2 (MH+): 817.6360, found 817.6372; FT-IR 3300–2700 (O-H stretch), 2960, 2793 (C-H stretch), 1479, 1453 (C=C aromatic stretch), 1234 (C-O stretch), 749, 734, 699 cm−1 (C-H out-of-plane deformation).
3.3. Single Crystal X-ray Diffraction
A single crystal of compound 4 was obtained from a concentrated chloroform solution. X-ray single-crystal structural data were collected on an Agilent Xcalibur diffractometer equipped with a fine-focus sealed tube X-ray source with graphite monochromated Mo-Kα radiation (λ = 0.71073 Å). The program CrysAlis PRO [27] was used for the data collection, cell refinement and data reduction. The structure was solved by SHELXS97 [28] and refined by the full-matrix least-squares method using SHELXL [29]. Refinement of F2 was done for all reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The structure was solved by direct methods. The asymmetric unit contains half the molecule (inversion symmetry). All non H-atoms are refined anisotropically. The H-atoms are placed in calculated positions except for H1, which was found. The thermal parameter was fixed to 1.2 times that of the parent atom and the O1–H1 bond length is restrained (DFIX) to 0.95 A. Crystal Data for C54H80N4O2 (M = 817.22 g/mol): triclinic, space group P-1 (no. 2), a = 8.4026(6) Å, b = 11.8152(8) Å, c = 13.3515(10) Å, α = 109.180(7)°, β = 98.602(6)°,γ = 97.535(6)°, V = 1214.57(15) Å3, Z = 1, T = 123 K, μ(MoKα) = 0.07 mm−1, Dcalc = 1.117 g/cm3, 9706 reflections measured (3.16° ≤ 2θ ≤ 25.0°), 4248 unique (Rint = 0.0378, Rsigma = 0.0576) which were used in all calculations. The final R1 was 0.062 (I > 2σ(I)) and wR2 was 0.136 (all data). Further details referring to the x-ray diffraction analysis are provided in the SI.
4. Conclusions
The title compound was successfully synthesized and characterized via spectroscopic techniques and single crystal X-ray diffraction. The macrocyclic ligand synthesized crystallizes in the triclinic space group P-1, with the asymmetric unit containing one-half of the molecule. The 14-membered macrocycle adopts a rectangular conformation, which according to Dale’s nomenclature is (3,4,3,4)-B. The structure is stabilized by hydrogen-bonding, which exists between the phenolic protons and the nitrogen atoms of the macrocyclic ring. Cyclic voltammetry of the ligand revealed a single quasi-reversible peak at −0.58 V (Epc = −0.48 V and Epa = −0.68 V) which is due to the electrochemical oxidation of the phenol to the phenoxyl radical.
Supplementary Materials
The following are available online at www.mdpi.com/1422-8599/2017/4/M963, Tables S1–S7: X-ray diffraction data for compound 4. “CCDC 1574392” also contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html.
Acknowledgments
R.E.M would like to thank Simon Duckett for his generosity relating to Schlenk line equipment and Manchester Metropolitan University for financial assistance. A.M.J would like to like to thank the Royal Society (RG150135).
Author Contributions
S.K.L., A.M.J. and R.E.M. conceived and designed the experiments; A.I.A.L. and M.K. performed the experiments; S.B. and S.K.L. performed the X-ray analysis; A.I.A.L. and R.E.M. analysed the data; R.E.M. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fricker, S.P. Physiology and Pharmacology of Plerixafor. Transfus. Med. Hemother. 2013, 40, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Poty, S.; Desogere, P.; Goze, C.; Boschetti, F.; D’Huys, T.; Schols, D.; Cawthorne, C.; Archibald, S.J.; Maecke, H.R.; Denat, F. New AMD3100 derivatives for CXCR4 chemokine receptor targeted molecular imaging studies: Synthesis, anti-HIV-1 evaluation and binding affinities. Dalton Trans. 2015, 44, 5004–5016. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.; Choi, J.-H.; Hunter, T.M.; Pannecouque, C.; Moggach, S.A.; Parsons, S.; De Clercq, E.; Sadler, P.J. Zinc(II) complexes of constrained antiviral macrocycles. Dalton Trans. 2012, 41, 6408–6418. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Huskens, D.; Daelemans, D.; Mewis, R.E.; Garcia, C.D.; Cain, A.N.; Freeman, T.N.C.; Pannecouque, C.; De Clercq, E.; Schols, D.; et al. CXCR4 chemokine receptor antagonists: Nickel(II) complexes of configurationally restricted macrocycles. Dalton Trans. 2012, 41, 11369–11377. [Google Scholar] [CrossRef] [PubMed]
- Blahut, J.; Hermann, P.; Galisova, A.; Herynek, V.; Cisarova, I.; Tosner, Z.; Kotek, J. Nickel(II) complexes of N-CH2CF3 cyclam derivatives as contrast agents for F-19 magnetic resonance imaging. Dalton Trans. 2016, 45, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Poty, S.; Gourni, E.; Desogere, P.; Boschetti, F.; Goze, C.; Maecke, H.R.; Denat, F. AMD3100: A Versatile Platform for CXCR4 Targeting Ga-68-Based Radiopharmaceuticals. Bioconjugate Chem. 2016, 27, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Holland, J.P.; Kenton, N.; Rotile, N.; Caravan, P. Macrocycle-Based Hydroxamate Ligands for Complexation and Immunoconjugation of 89Zirconium for Positron Emission Tomography (PET) Imaging. Chempluschem 2016, 81, 274–281. [Google Scholar] [CrossRef] [PubMed]
- David, T.; Kubicek, V.; Gutten, O.; Lubal, P.; Kotek, J.; Pietzsch, H.-J.; Rulisek, L.; Hermann, P. Cyclam Derivatives with a Bis(phosphinate) or a Phosphinato-Phosphonate Pendant Arm: Ligands for Fast and Efficient Copper(II) Complexation for Nuclear Medical Applications. Inorg. Chem. 2015, 54, 11751–11766. [Google Scholar] [CrossRef] [PubMed]
- Halime, Z.; Frindel, M.; Camus, N.; Orain, P.-Y.; Lacombe, M.; Cherel, M.; Gestin, J.-F.; Faivre-Chauvet, A.; Tripier, R. New synthesis of phenyl-isothiocyanate C-functionalised cyclams. Bioconjugation and Cu-64 phenotypic PET imaging studies of multiple myeloma with the te2a derivative. Org. Biomol. Chem. 2015, 13, 11302–11314. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Lv, K.; Zhong, W.; Wang, H.; Wu, Z.; Yi, P.; Jiang, J. Cyclam-functionalized carbon dots sensor for sensitive and selective detection of copper(II) ion and sulfide anion in aqueous media and its imaging in live cells. Sens. Actuators B 2016, 224, 298–306. [Google Scholar] [CrossRef]
- Feier, B.; Fizesan, I.; Meriadec, C.; Girard, S.A.; Cristea, C.; Sandulescu, R.; Geneste, F. Influence of the electrografting method on the performances of a flow electrochemical sensor using modified electrodes for trace analysis of copper (II). J. Electroanal. Chem. 2015, 744, 1–7. [Google Scholar] [CrossRef]
- Jasmin, J.-P.; Ouhenia-Ouadahi, K.; Miserque, F.; Dumas, E.; Cannizzo, C.; Chausse, A. Straightforward grafting approach for cyclam-functionalized screen-printed electrodes for selective Cu(II) determination. Electrochim. Acta 2016, 200, 115–122. [Google Scholar] [CrossRef]
- Pandya, D.N.; Bhatt, N.; An, G.I.; Ha, Y.S.; Soni, N.; Lee, H.; Lee, Y.J.; Kim, J.Y.; Lee, W.; Ahn, H.; Yoo, J. Propylene Cross-Bridged Macrocyclic Bifunctional Chelator: A New Design for Facile Bioconjugation and Robust 64Cu Complex Stability. J. Med. Chem. 2014, 57, 7234–7243. [Google Scholar] [CrossRef] [PubMed]
- Maria, L.; Santos, I.C.; Alves, L.G.; Marçalo, J.; Martins, A.M. Rare earth metal complexes anchored on a new dianionic bis(phenolate)dimethylamineCyclam ligand. J. Organomet. Chem. 2013, 728, 57–67. [Google Scholar] [CrossRef]
- Maria, L.; Santos, I.C.; Sousa, V.R.; Marçalo, J. Uranium(III) Redox Chemistry Assisted by a Hemilabile Bis(phenolate) Cyclam Ligand: Uranium–Nitrogen Multiple Bond Formation Comprising a trans-{RN═U(VI)═NR}2+ Complex. Inorg. Chem. 2015, 54, 9115–9126. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.A.; Fanwick, P.E.; Welch, M.J. Synthesis, characterization, and solid-state structure of a new hexachelating ligand and its complex with gallium(III). Inorg. Chem. 1989, 28, 1504–1506. [Google Scholar] [CrossRef]
- Auerbach, U.; Eckert, U.; Wieghardt, K.; Nuber, B.; Weiss, J. Synthesis and coordination chemistry of the hexadentate ligands 1,4,7-tris(2-hydroxybenzyl)-1,4,7-triazacyclononane (H3L1) and 1,4,7-tris(3-tert-butyl-2-hydroxybenzyl)-1,4,7-triazacyclononane (H3L2). Crystal structures of [HL1CuII] and [L2FeIII]acacH. Inorg. Chem. 1990, 29, 938–944. [Google Scholar] [CrossRef]
- Kimura, S.; Bill, E.; Bothe, E.; Weyhermüller, T.; Wieghardt, K. Phenylthiyl Radical Complexes of Gallium(III), Iron(III), and Cobalt(III) and Comparison with Their Phenoxyl Analogues. J. Am. Chem. Soc. 2001, 123, 6025–6039. [Google Scholar] [CrossRef] [PubMed]
- Pujols-Ayala, I.; Barry, B.A. Tyrosyl radicals in Photosystem II. Biochim. Biophys. Acta Bioenerg. 2004, 1655, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.S.; Dooley, D.M. Copper-tyrosyl radical enzymes. Curr. Opin. Chem. Biol. 2003, 7, 189–196. [Google Scholar] [CrossRef]
- Royal, G.; Dahaoui-Gindrey, V.; Dahaoui, S.; Tabard, A.; Guilard, R.; Pullumbi, P.; Lecomte, C. New Synthesis of trans-Disubstituted Cyclam Macrocycles—Elucidation of the Disubstitution Mechanism on the Basis of X-ray Data and Molecular Modeling. Eur. J. Org. Chem. 1998, 1998, 1971–1975. [Google Scholar] [CrossRef]
- Alves, L.G.; Duarte, M.T.; Martins, A.M. Structural features of neutral and cationic cyclams. J. Mol. Struct. 2015, 1098, 277–288. [Google Scholar] [CrossRef]
- Dale, J. Exploratory Calculations of Medium and Large Rings. Part 1. Conformational Minima of Cycloalkanes. Acta Chem. Scand. 1973, 27, 1115–1129. [Google Scholar] [CrossRef]
- Meyer, M.; Dahaoui-Gindrey, V.; Lecomte, C.; Guilard, R. Conformations and coordination schemes of carboxylate and carbamoyl derivatives of the tetraazamacrocycles cyclen and cyclam, and the relation to their protonation states. Coord. Chem. Rev. 1998, 178, 1313–1405. [Google Scholar] [CrossRef]
- Bosnich, B.; Poon, C.K.; Tobe, M.L. Complexes of Cobalt(III) with a Cyclic Tetradentate Secondary Amine. Inorg. Chem. 1965, 4, 1102–1108. [Google Scholar] [CrossRef]
- Maria, L.; Sousa, V.R.; Santos, I.C.; Mora, E.; Marçalo, J. Synthesis and structural characterization of polynuclear divalent ytterbium complexes supported by a bis(phenolate) cyclam ligand. Polyhedron 2016, 119, 277–285. [Google Scholar] [CrossRef]
- CrysAlis P R O. Agilent Technologies, Yarnton, England; RC Clark, JS Reid. Acta Crystallogr. 1995, 51, 887. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).