Abstract
A new member of the 2-acetylpyridine family has been prepared and characterized. Its synthesis is a two-step process starting from a pyridyl-alcohol in which the ketone moiety is protected as a cyclic acetal. Alkylation of the alcohol followed by hydrolysis of the acetal afforded the title compound in 52% overall yield.
1. Introduction
2-Acetylpyridine derivatives are interesting synthetic intermediates. For example, such compounds have been used as starting materials in the preparation of potential anti-cancer agents [1,2,3], of complexes with anti-microbial properties [4,5], of pyridinyl-pyrimidines [6], of catalysts [7,8,9], materials for water treatment [10] or molecules possessing magnetic properties [11] just to name a few.
Furthermore, 2-acetylpyridine have been widely used in Kröhnke’s synthesis [12,13] of terpyridines [14,15,16], which are increasingly used in many applications [17]. As a consequence, the development of new 2-acetylpyridine derivatives is of great interest in view of the broad range of possible applications for these molecules.
This paper described the preparation of 1-{4-[(hexyloxy)methyl]pyridin-2-yl}ethanone, which is one member of this 2-acetylpyridine family.
2. Results and Discussion
The synthesis of 1-{4-[(hexyloxy)methyl]pyridin-2-yl}ethanone (3) is a two-step process, starting from alcohol derivative (1), which can be obtained either from methyl or ethyl 2-acetylisonicotinate [18,19,20] according to a procedure described in the literature [21] (Scheme 1).
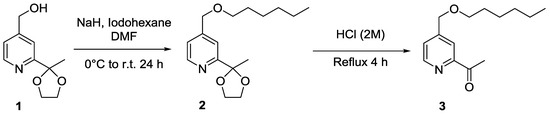
Scheme 1.
Synthetic route to 1-{4-[(hexyloxy)methyl]pyridin-2-yl}ethanone.
Intermediate 4-[(hexyloxy)methyl]-2-(2-methyl-1,3-dioxolan-2-yl)pyridine (2) (Figure 1) [22] was obtained by O-alkylation of 1 with iodohexane. Infrared (IR) spectra of 2 indicate the disappearance of the O–H valence vibration band at 3607 cm−1, while new C–H vibrations bands from the hexyl chain arise between 2959 and 2861 cm−1, and account for ether formation.
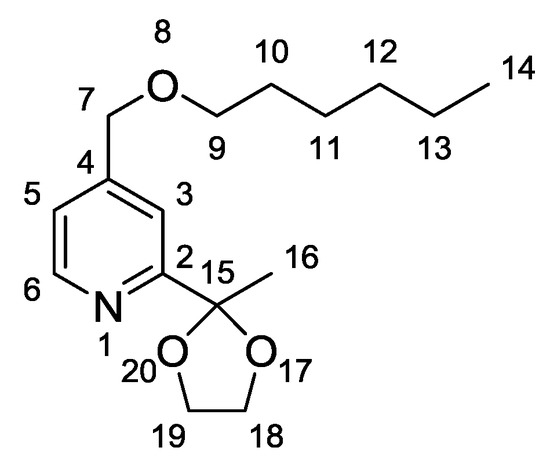
Figure 1.
Structure and atom numbering of Intermediate Compound 2.
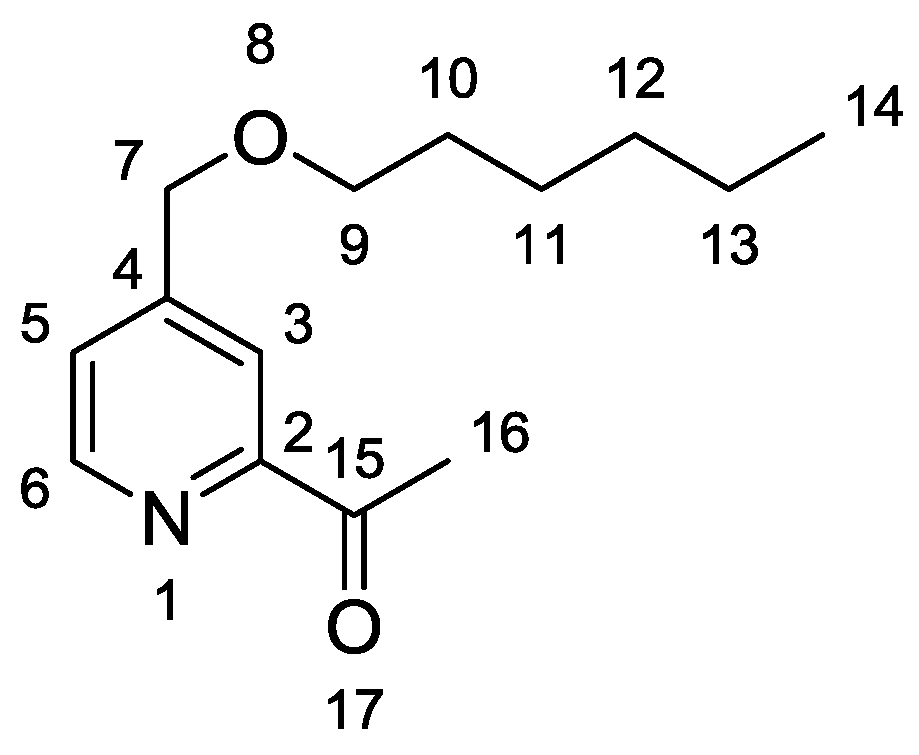
Deprotection of the ketone function was carried out in an acidic aqueous solution under reflux. Compound 3 was obtained with an overall yield of 52% over the aforementioned two steps. Regarding the IR spectra, a new valence vibration band at 1697 cm−1, corresponding to the ketone group (C=O vibration), was observed. With regard to 1H-NMR spectra, an intense magnetic deshielding was detected with respect to protons of the methyl ketone group in 3 (H16, δ = 2.73 ppm) compared with the same protons on Compound 2 (H16, δ = 1.66 ppm). The 13C-NMR spectrum confirms the presence of a new peak at 200.0 ppm, which can be attributed to the carbon of the ketone function.
3. Materials and Methods
All reagents were purchased from commercial suppliers and used as received. Flash chromatography was carried out on a Combiflash Rf+ Lumen (Teledyne ISCO, Lincoln, NE, USA) using 80 g silica column from Macherey-Nagel. 1H and 13C-NMR spectra were recorded on a Bruker AC 400 (Bruker, Wissembourg, France) at 400 and 100 MHz, respectively, using CDCl3 as a solvent. IR spectra were recorded as dichloromethane solutions (C = 0.055 mol·L−1) on an IR Affinity spectrometer (Shimadzu, Kyoto, Japan). Elemental analyses were performed at Service d’Analyses Elementaires, UMR 7565 CNRS, Vandoeuvre-les-Nancy, France.
1-{4-[(Hexyloxy)methyl]pyridin-2-yl}ethan-1-one (3)
4-(hydroxymethyl)-2-(2-methyl-1,3-dioxolan-2-yl)pyridine (1) [21] (1.38 g, 7.07 mmol, 1 equiv) was dissolved in 20 mL of DMF. The mixture was cooled at 0 °C, NaH (60%) (7.78 mmol, 1.1 equiv) was added, and the mixture was stirred for 30 min. Iodohexane was added (1.15 mL, 7.78 mmol, 1.1 equiv), and the mixture was stirred at room temperature for 24 h. One hundred fifty milliliters of water was added, and the aqueous layer was extracted with ethyl acetate (3 × 50 mL). The combined organic layers were washed with water (2 × 40 mL), brine (40 mL), dried over Na2SO4, and filtered, and the solvent was evaporated. Purification by flash chromatography (eluent: hexane/ethyl acetate: 75/25) yielded 2 as a colorless oil (1.34 g, 68%).
Compound 2 (4.16 g, 14.9 mmol, 1 equiv) was added with aqueous HCl (2M, 30 mL). The mixture was stirred at reflux for 4 h. After cooling occurred, a saturated solution of Na2CO3 (30 mL) was added, and the aqueous layer was extracted with dichloromethane (3 × 40 mL). The combined organic layers were washed with brine (40 mL), dried over Na2SO4, and filtered, and the solvent was evaporated. Purification by flash chromatography (eluent: hexane/ethyl acetate: 90/10 to 70/30) yielded 3 as a colorless oil (2.65 g, 76%). 1H-NMR (CDCl3, 400 MHz), δ (ppm) = 8.65 (d, 1H6, J = 4.9 Hz), 7.98 (s, 1H3), 7.49 (d, 1H5, J = 4.9 Hz), 4.57 (s, 2H7), 3.52 (t, 2H9, J = 6.6 Hz), 2.73 (s, 3H16), 1.65 (quint, 2H10, J = 7.0 Hz), 1.31–1.04 (m, 6H11–13), 0.89 (t, 3H14, J = 6.6 Hz). 13C-NMR (CDCl3, 100 MHz), δ (ppm) = 200.0, 153.5; 149.5; 149.0; 125.1; 119.8; 71.4; 70.9; 31.6; 29.6; 25.9; 25.8; 22.6; 14.0. IR 3052, 3007, 2961, 2932, 2861, 1697, 1603 cm−1. Elemental analysis for C14H21NO2: C, 71.46; H, 8.99; N, 5.95. Found C, 71.46; H, 8.99; N, 5.89.
Supplementary Materials
The following are available online: 1H-NMR, 13C-NMR, IR spectra, elemental analyses reports and FIDs for Compounds (2) and (3).
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Région Franche-Comté is gratefully acknowledged for financial support.
Author Contributions
Florian Charrier, Jérôme Husson, and Laurent Guyard conceived and designed the experiments. Florian Charrier performed the experiments. All authors contributed to data analysis and contributed to the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Pape, V.F.S.; Toth, S.; Füredi, A.; Szebényi, K.; Lovrics, A.; Szabo, P.; Wiese, M.; Szakacs, G. Design, synthesis and biological evaluation of thiosemicarbazones, hydrazinobenzothiazoles and arylhydrazones as anticancer agents with a potential to overcome multidrug resistance. Eur. J. Med. Chem. 2016, 117, 335–354. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-Q.; Mao, X.-J.; Jia, L.; Xu, J.; Zhu, T.-F.; Cai, H.-X.; Bie, H.-Y.; Chen, R.-H.; Ma, T.-L. Synthesis, characterization and anticancer activities of two lanthanide(III) complexes with a nicotinohydrazone ligand. J. Mol. Struct. 2015, 1102, 86–90. [Google Scholar] [CrossRef]
- Zhang, N.; Fan, Y.; Zhang, Z.; Zuo, J.; Zang, P.; Wang, Q.; Liu, S.; Bi, C. Syntheses, crystal structures and anticancer activities of three novel transition metal complexes with Schiff base derived from 2-acetylpyridine and l-tryptophan. Inorg. Chem. Commun. 2012, 22, 68–72. [Google Scholar] [CrossRef]
- Vojinovic-Jesic, L.; Radanovic, M.M.; Rodic, M.V.; Zivkovic-Radovanovic, V.; Jovanovic, L.S.; Leovac, V.M. Syntheses and characterization of 2-acetylpyridine-aminoguanidine and its copper(II) complexes: Crystallographic and antimicrobial study. Polyhedron 2016, 117, 526–534. [Google Scholar] [CrossRef]
- Ferreira, I.P.; Pilo, E.D.L.; Recio-Despaigne, A.A.; Da Silva, J.G.; Ramos, J.P.; Marques, L.B.; Prazeres, P.H.D.M.; Takahashi, J.A.; Souza-Fagundes, E.M.; Rocha, W.; et al. Bismuth(III) complexes with 2-acetylpyridine- and 2-benzoylpyridine-derived hydrazones: Antimicrobial and cytotoxic activities and effects on the clonogenic survival of human solid tumor cells. Bioorg. Med. Chem. 2016, 24, 2988–2998. [Google Scholar] [CrossRef] [PubMed]
- Dahmann, G.; Gen, D.; Fleck, M.; Hoffmann, M.; Klicic, J.; East, S.P.; Napier, S.; Scott, J. Substituted Pyridinyl-Pyrimidines and Their Use As Medicaments. Patent WO2012101013A1, 2 August 2012. [Google Scholar]
- Sharma, R.K.; Monga, Y. Silica encapsulated magnetic nanoparticles-supported Zn(II) nanocatalyst: A versatile integration of excellent reactivity and selectivity for the synthesis of azoxyarenes, combined with facile catalyst recovery and recyclability. Applied Catal. A Gen. 2013, 454, 1–10. [Google Scholar] [CrossRef]
- Suganthy, P.K.; Prabhu, R.N.; Sridevi, V.S. Synthesis, structural characterization and catalytic transfer hydrogenation of ruthenium(II) carbonyl complexes bearing N,N,O pincer type benzoylhydrazone ligands. Polyhedron 2015, 88, 57–62. [Google Scholar] [CrossRef]
- Maurya, M.R.; Chaudhary, N.; Kumar, A.; Avecilla, F.; Pessoa, J.C. Polystyrene bound dioxidovanadium(V) complexes of 2-acetylpyridine derived ligands for catalytic oxidations. Inorg. Chim. Acta 2014, 420, 24–38. [Google Scholar] [CrossRef]
- Ceglowski, M.; Schroeder, G. Preparation of porous resin with Schiff base chelating groups for removal of heavy metal ions from aqueous solutions. Chem. Eng. J. 2015, 263, 402–411. [Google Scholar] [CrossRef]
- Nakanishi, T.; Sato, O. Synthesis, Structure, and Magnetic Properties of New Spin Crossover Fe(II) Complexes Forming Short Hydrogen Bonds with Substituted Dicarboxylic Acids. Crystals 2016, 6, 131. [Google Scholar] [CrossRef]
- Kröhnke, F. The Specific Synthesis of Pyridines and Oligopyridines. Synthesis 1976, 1976, 1–24. [Google Scholar] [CrossRef]
- Sasaki, I. Recent Uses of Kröhnke Methodology: A Short Survey. Synthesis 2016, 48, 1974–1992. [Google Scholar] [CrossRef]
- Thompson, A.M.W.C. The Synthesis of 2,2′:6′,2″-Terpyridine Ligands—Versatile Building Blocks for Supramolecular Chemistry. Coord. Chem. Rev. 1997, 160, 1–52. [Google Scholar] [CrossRef]
- Heller, M.; Schubert, U.S. Syntheses of Functionalized 2,2′:6′,2′′-Terpyridines. Eur. J. Org. Chem. 2003, 2003, 947–961. [Google Scholar] [CrossRef]
- Fallahpour, R.-A. Synthesis of 4′-Substituted-2,2′:6′,2′′-Terpyridines. Synthesis 2003, 2, 0155–0184. [Google Scholar] [CrossRef]
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Mikel, C.; Potvin, P.G. Synthesis and enhanced photosensitization ability of a 4-carboxy-2,2′:6′,2′′-terpyridine complex of ruthenium(II). Polyhedron 2002, 21, 49–54. [Google Scholar] [CrossRef]
- Ishihara, M.; Tsuneya, T.; Shiga, M.; Kawashima, S.; Yamagishi, K.; Yoshida, F.; Sato, H.; Uneyama, K. New Pyridine Derivatives and Basic Components in Spearmint Oil (Mentha gentilis f. cardiaca) and Peppermint Oil (Mentha piperita). J. Agric. Food Chem. 1992, 40, 1647–1655. [Google Scholar] [CrossRef]
- Dehaudt, J.; Husson, J.; Guyard, L. A more efficient synthesis of 4,4′,4′′-tricarboxy-2,2′:6′,2′′-terpyridine. Green Chem. 2011, 13, 3337–3340. [Google Scholar] [CrossRef]
- Jacques, A.; Cerfontaine, S.; Elias, B. Access to Functionalized Luminescent Multi-2,2′:6′,2″-Terpyridine Ligands. J. Org. Chem. 2015, 80, 11143–11148. [Google Scholar] [CrossRef] [PubMed]
- Characterisation data for (2): 1H-NMR (CDCl3, 400 MHz), δ(ppm) = 8.52 (d, 1H6, J = 5.0 Hz), 7.43 (s, 1H3), 7.14 (dd, 1H5, J = 5.0 Hz, 0.8 Hz), 4.45 (s, 2H7), 4.04–4.00 (m, 2H18), 3.82–3.79 (m, 2H19), 3.44 (t, 2H9, J = 6.6 Hz), 1.66 (s, 3H16), 1.57 (quint, 2H10, J = 7.0 Hz), 1.34–1.23 (m, 6H11–13), 0.82 (t, 3H14, J = 6.9 Hz). 13C-NMR (CDCl3, 100 MHz), δ(ppm) = 161.0; 149.4; 148.8; 121.0; 117.5; 108.6; 71.3; 71.3; 64.9; 31.6; 25.8; 25.4; 22.6. IR 3068, 3042, 2959, 2934, 2861, 1605 cm−1. Elemental Analysis for C16H25NO3: C, 68.79; H, 9.02; N, 5.01. Found C, 68.30; H, 8.91; N, 5.07.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).