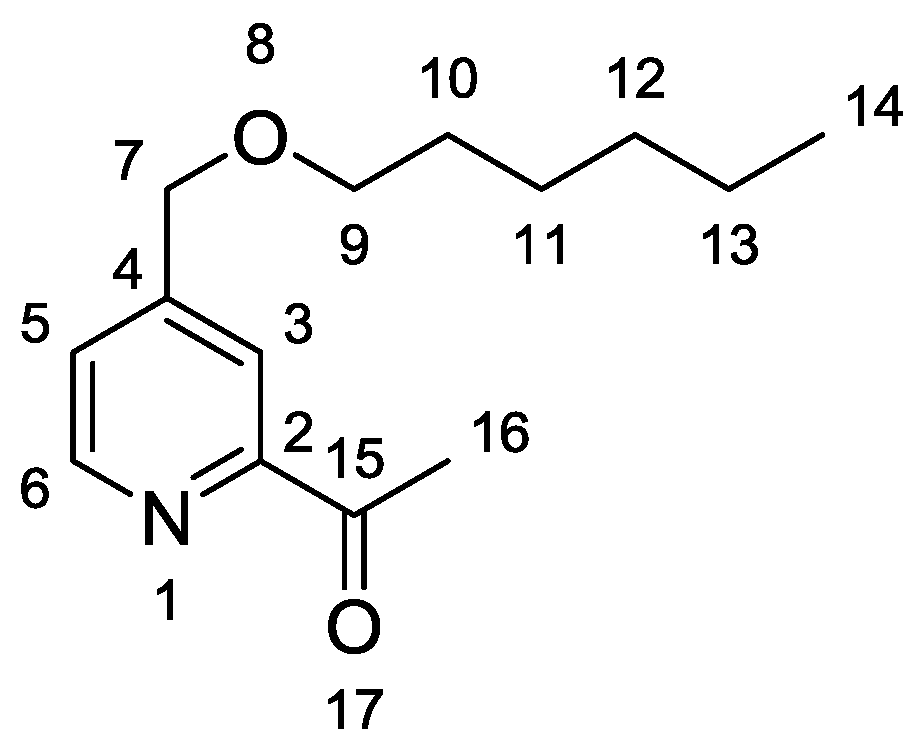
1-{4-[(Hexyloxy)methyl]pyridin-2-yl}ethanone
Abstract
:1. Introduction
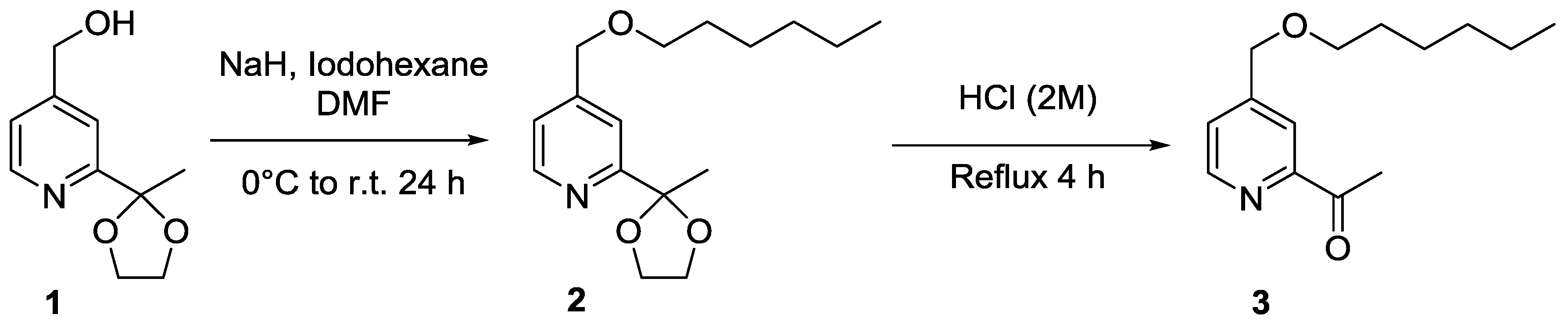
2. Results and Discussion
3. Materials and Methods
1-{4-[(Hexyloxy)methyl]pyridin-2-yl}ethan-1-one (3)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Pape, V.F.S.; Toth, S.; Füredi, A.; Szebényi, K.; Lovrics, A.; Szabo, P.; Wiese, M.; Szakacs, G. Design, synthesis and biological evaluation of thiosemicarbazones, hydrazinobenzothiazoles and arylhydrazones as anticancer agents with a potential to overcome multidrug resistance. Eur. J. Med. Chem. 2016, 117, 335–354. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-Q.; Mao, X.-J.; Jia, L.; Xu, J.; Zhu, T.-F.; Cai, H.-X.; Bie, H.-Y.; Chen, R.-H.; Ma, T.-L. Synthesis, characterization and anticancer activities of two lanthanide(III) complexes with a nicotinohydrazone ligand. J. Mol. Struct. 2015, 1102, 86–90. [Google Scholar] [CrossRef]
- Zhang, N.; Fan, Y.; Zhang, Z.; Zuo, J.; Zang, P.; Wang, Q.; Liu, S.; Bi, C. Syntheses, crystal structures and anticancer activities of three novel transition metal complexes with Schiff base derived from 2-acetylpyridine and l-tryptophan. Inorg. Chem. Commun. 2012, 22, 68–72. [Google Scholar] [CrossRef]
- Vojinovic-Jesic, L.; Radanovic, M.M.; Rodic, M.V.; Zivkovic-Radovanovic, V.; Jovanovic, L.S.; Leovac, V.M. Syntheses and characterization of 2-acetylpyridine-aminoguanidine and its copper(II) complexes: Crystallographic and antimicrobial study. Polyhedron 2016, 117, 526–534. [Google Scholar] [CrossRef]
- Ferreira, I.P.; Pilo, E.D.L.; Recio-Despaigne, A.A.; Da Silva, J.G.; Ramos, J.P.; Marques, L.B.; Prazeres, P.H.D.M.; Takahashi, J.A.; Souza-Fagundes, E.M.; Rocha, W.; et al. Bismuth(III) complexes with 2-acetylpyridine- and 2-benzoylpyridine-derived hydrazones: Antimicrobial and cytotoxic activities and effects on the clonogenic survival of human solid tumor cells. Bioorg. Med. Chem. 2016, 24, 2988–2998. [Google Scholar] [CrossRef] [PubMed]
- Dahmann, G.; Gen, D.; Fleck, M.; Hoffmann, M.; Klicic, J.; East, S.P.; Napier, S.; Scott, J. Substituted Pyridinyl-Pyrimidines and Their Use As Medicaments. Patent WO2012101013A1, 2 August 2012. [Google Scholar]
- Sharma, R.K.; Monga, Y. Silica encapsulated magnetic nanoparticles-supported Zn(II) nanocatalyst: A versatile integration of excellent reactivity and selectivity for the synthesis of azoxyarenes, combined with facile catalyst recovery and recyclability. Applied Catal. A Gen. 2013, 454, 1–10. [Google Scholar] [CrossRef]
- Suganthy, P.K.; Prabhu, R.N.; Sridevi, V.S. Synthesis, structural characterization and catalytic transfer hydrogenation of ruthenium(II) carbonyl complexes bearing N,N,O pincer type benzoylhydrazone ligands. Polyhedron 2015, 88, 57–62. [Google Scholar] [CrossRef]
- Maurya, M.R.; Chaudhary, N.; Kumar, A.; Avecilla, F.; Pessoa, J.C. Polystyrene bound dioxidovanadium(V) complexes of 2-acetylpyridine derived ligands for catalytic oxidations. Inorg. Chim. Acta 2014, 420, 24–38. [Google Scholar] [CrossRef]
- Ceglowski, M.; Schroeder, G. Preparation of porous resin with Schiff base chelating groups for removal of heavy metal ions from aqueous solutions. Chem. Eng. J. 2015, 263, 402–411. [Google Scholar] [CrossRef]
- Nakanishi, T.; Sato, O. Synthesis, Structure, and Magnetic Properties of New Spin Crossover Fe(II) Complexes Forming Short Hydrogen Bonds with Substituted Dicarboxylic Acids. Crystals 2016, 6, 131. [Google Scholar] [CrossRef]
- Kröhnke, F. The Specific Synthesis of Pyridines and Oligopyridines. Synthesis 1976, 1976, 1–24. [Google Scholar] [CrossRef]
- Sasaki, I. Recent Uses of Kröhnke Methodology: A Short Survey. Synthesis 2016, 48, 1974–1992. [Google Scholar] [CrossRef]
- Thompson, A.M.W.C. The Synthesis of 2,2′:6′,2″-Terpyridine Ligands—Versatile Building Blocks for Supramolecular Chemistry. Coord. Chem. Rev. 1997, 160, 1–52. [Google Scholar] [CrossRef]
- Heller, M.; Schubert, U.S. Syntheses of Functionalized 2,2′:6′,2′′-Terpyridines. Eur. J. Org. Chem. 2003, 2003, 947–961. [Google Scholar] [CrossRef]
- Fallahpour, R.-A. Synthesis of 4′-Substituted-2,2′:6′,2′′-Terpyridines. Synthesis 2003, 2, 0155–0184. [Google Scholar] [CrossRef]
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Mikel, C.; Potvin, P.G. Synthesis and enhanced photosensitization ability of a 4-carboxy-2,2′:6′,2′′-terpyridine complex of ruthenium(II). Polyhedron 2002, 21, 49–54. [Google Scholar] [CrossRef]
- Ishihara, M.; Tsuneya, T.; Shiga, M.; Kawashima, S.; Yamagishi, K.; Yoshida, F.; Sato, H.; Uneyama, K. New Pyridine Derivatives and Basic Components in Spearmint Oil (Mentha gentilis f. cardiaca) and Peppermint Oil (Mentha piperita). J. Agric. Food Chem. 1992, 40, 1647–1655. [Google Scholar] [CrossRef]
- Dehaudt, J.; Husson, J.; Guyard, L. A more efficient synthesis of 4,4′,4′′-tricarboxy-2,2′:6′,2′′-terpyridine. Green Chem. 2011, 13, 3337–3340. [Google Scholar] [CrossRef]
- Jacques, A.; Cerfontaine, S.; Elias, B. Access to Functionalized Luminescent Multi-2,2′:6′,2″-Terpyridine Ligands. J. Org. Chem. 2015, 80, 11143–11148. [Google Scholar] [CrossRef] [PubMed]
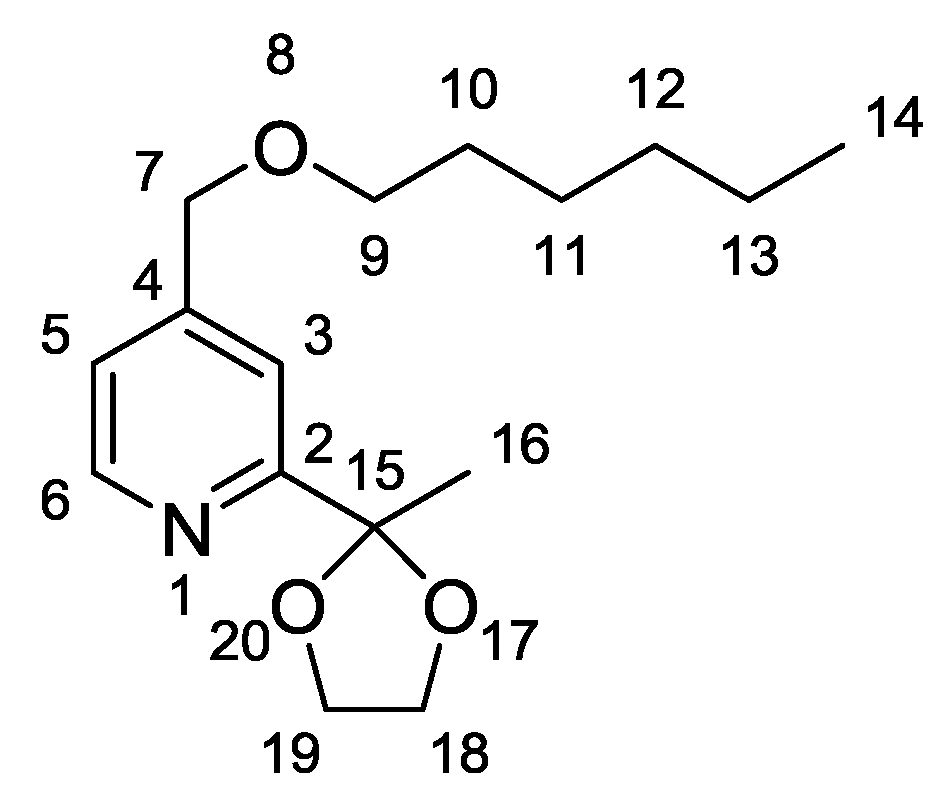
- Characterisation data for (2): 1H-NMR (CDCl3, 400 MHz), δ(ppm) = 8.52 (d, 1H6, J = 5.0 Hz), 7.43 (s, 1H3), 7.14 (dd, 1H5, J = 5.0 Hz, 0.8 Hz), 4.45 (s, 2H7), 4.04–4.00 (m, 2H18), 3.82–3.79 (m, 2H19), 3.44 (t, 2H9, J = 6.6 Hz), 1.66 (s, 3H16), 1.57 (quint, 2H10, J = 7.0 Hz), 1.34–1.23 (m, 6H11–13), 0.82 (t, 3H14, J = 6.9 Hz). 13C-NMR (CDCl3, 100 MHz), δ(ppm) = 161.0; 149.4; 148.8; 121.0; 117.5; 108.6; 71.3; 71.3; 64.9; 31.6; 25.8; 25.4; 22.6. IR 3068, 3042, 2959, 2934, 2861, 1605 cm−1. Elemental Analysis for C16H25NO3: C, 68.79; H, 9.02; N, 5.01. Found C, 68.30; H, 8.91; N, 5.07.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charrier, F.; Husson, J.; Guyard, L. 1-{4-[(Hexyloxy)methyl]pyridin-2-yl}ethanone. Molbank 2017, 2017, M940. https://doi.org/10.3390/M940
Charrier F, Husson J, Guyard L. 1-{4-[(Hexyloxy)methyl]pyridin-2-yl}ethanone. Molbank. 2017; 2017(2):M940. https://doi.org/10.3390/M940
Chicago/Turabian StyleCharrier, Florian, Jérôme Husson, and Laurent Guyard. 2017. "1-{4-[(Hexyloxy)methyl]pyridin-2-yl}ethanone" Molbank 2017, no. 2: M940. https://doi.org/10.3390/M940
APA StyleCharrier, F., Husson, J., & Guyard, L. (2017). 1-{4-[(Hexyloxy)methyl]pyridin-2-yl}ethanone. Molbank, 2017(2), M940. https://doi.org/10.3390/M940






