Abstract
Methyl 2,5-dihydroxy-4-(3′-methyl-2′-butenyl)benzoate (1) was isolated from the root of Erythrina subumbrans. The chemical structure of 1 has been elucidated based on spectroscopy UV-Vis, HRESIMS, 1D and 2D NMR analysis.
1. Introduction
The genus Erythrina (Euphorbiaceae) comprises more than 100 species that are widely distributed in tropical and subtropical regions. The evergreen plants of Erythrina occur in almost every part of Indonesia, from Sumatra to Irian and the plants is commonly known as ‘dadap’. Many of these species are used indigenously as traditional medicines to treat various diseases, such as infection, cough, malaria, inflammation, and asthma. This genus has been shown to produce a number of phenolic compounds, particularly alkaloids [1], flavonoids [2,3], pterocarpans [4,5] and stilbenoids [6]. In continuation of our research into the phenolic compound in this medicinal plant, we report the isolation of methyl 2,5-dihydroxy-4-(3′-methyl-2′-butenyl)benzoate (1) from the methanol extract of the the root of Erythrina subumbrans. The chemical structure of compound 1 was established by UV, HRESIMS, 1D and 2D NMR, as well as by comparison with those related compounds previously reported. The antioxidant activity against DPPH radical scavenging of the isolated compound 1 is also briefly described.
2. Result and Discussion
Extraction of the dried milled of roots of E. subumbrans (1.5 kg) was carried out using methanol, and then methanol extract was partitioned with n-hexane and ethyl acetate. The ethyl acetate extract (18 g) was separated by vacuum liquid chromatography on silica gel and radial chromatography yielded methyl 2,5-dihydroxy-4-(3′-methyl-2′-butenyl) benzoate 1 (Figure 1).
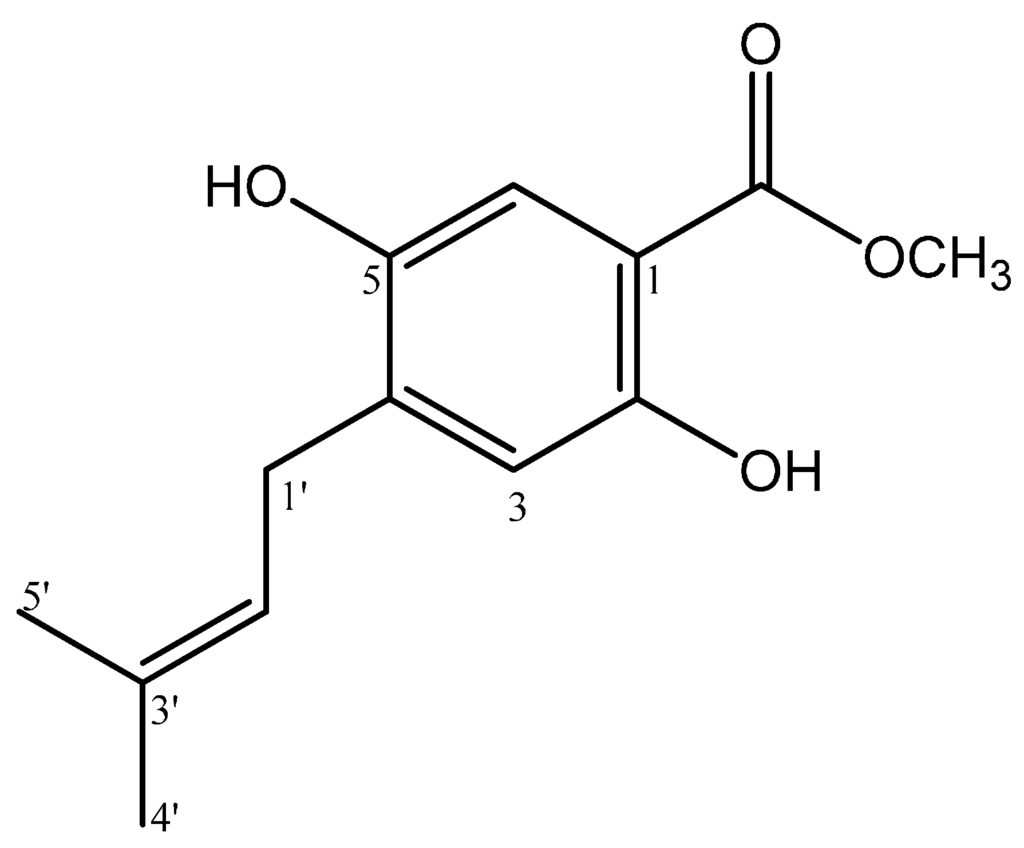
Figure 1.
Structure of methyl 2,5-dihydroxy-4-(3′-methyl-2′-butenyl)benzoate (1).
Methyl 2,5-dihydroxy-4-(3′-methyl-2′-butenyl)benzoate (1) was isolated as white solid. The UV spectrum exhibited absorption maxima λmaks 224 and 287 typical for a 4-methyl benzoate chromophore [7]. The HRESIMS spectrum showed a quasimolecular ion [M − H]− at m/z 235.0971 (calcd. 235.0970), which correspondend to the molecular formula of C13H15O4. The 1H-NMR (Table 1) spectrum of 1, the presence of two singlet aromatic proton signals at δH 7.56 (1H, s, H-6) and 6.39 (1H, s, H-3) suggest that compound 1 is typical for a methyl benzoate with three substituents [8]. In the 13C-NMR spectrum (Table 1), the results for 1 showed 13 carbon signals consistent for methyl isoprenylated benzoate structure, and two carbon signals at δC 52.0 and 170.4 were assigned to a methoxyl and carbonyl carbon from methyl benzoate structure. These spectroscopic data, therefore, suggested that 1 is a methyl benzoate containing an isoprenyl (3′-methyl-2′-butenyl) side chain. Furthermore, the presence of other two oxyaryl signals (δδC 160.9 and 162.1) indicated that the methyl benzoate has two hydroxyl groups. The side chain was deduced to be an isoprenyl group from the observation in the 1H-NMR spectrum (Table 1) of two methyl singlets (δH 1.76 and 1.77), one methylene signal (δH 3.27), and one methin vinyl signal (δH 5.28). The presence of a chelated –OH group at δH 10.79 gave the position of hydroxyl at C-2. The presence of long range correlations in the HMBC spectrum between the proton signal δH 10.79 with two quartenery carbon signals at δC 105.4 (C-1), 162.1 (C-2) and one methyne carbon at δC 103,4 (C-4) confirmed the -OH group (δH 10.79) attached at C-2. In the aromatic region of 1H-NMR spectrum, two singlet signals at δH 6.39 and 7.56 suggested that the position protons are found at H-3 and H-6. The presence of long range correlations in the HMBC spectrum of 1 between the proton signal aromatic at H-3 (δH 6.39) with four quarternary carbon signals at δC 105.4 (C-1), 162.1 (C-2), 119.2 (C-4), and 160.9 (C-5) unambiguously placed the isoprenyl at C-4 and hydroxyl groups at C-2 and C-5. The placement of isoprenyl group at C-4 suggested the correlation methylene signal (δH 3.27) with three quarternary carbon signals at δC 160.9 (C-5), 119.2 (C-4), 135.0 (C-3′), and two tertiery carbon signals at δC 103.4 (C-3), 121.6 (C-2′). Therefore, compound 1, was elucidated as methyl 2,5-dihydroxy-4-(3′-methyl-2′-butenyl)benzoate. Other HMBC correlations consistent with the structure 1 are shown in Table 1 and Figure 2. To our knowledge, compound 1 was the first example of methyl benzoate derivative in the genus Erythrina with an isoprenyl side chain.

Table 1.
NMR spectroscopic data of methyl 2,5-dihydroxy-4-(3′-methyl-2′-butenyl)benzoate in CDCl3.
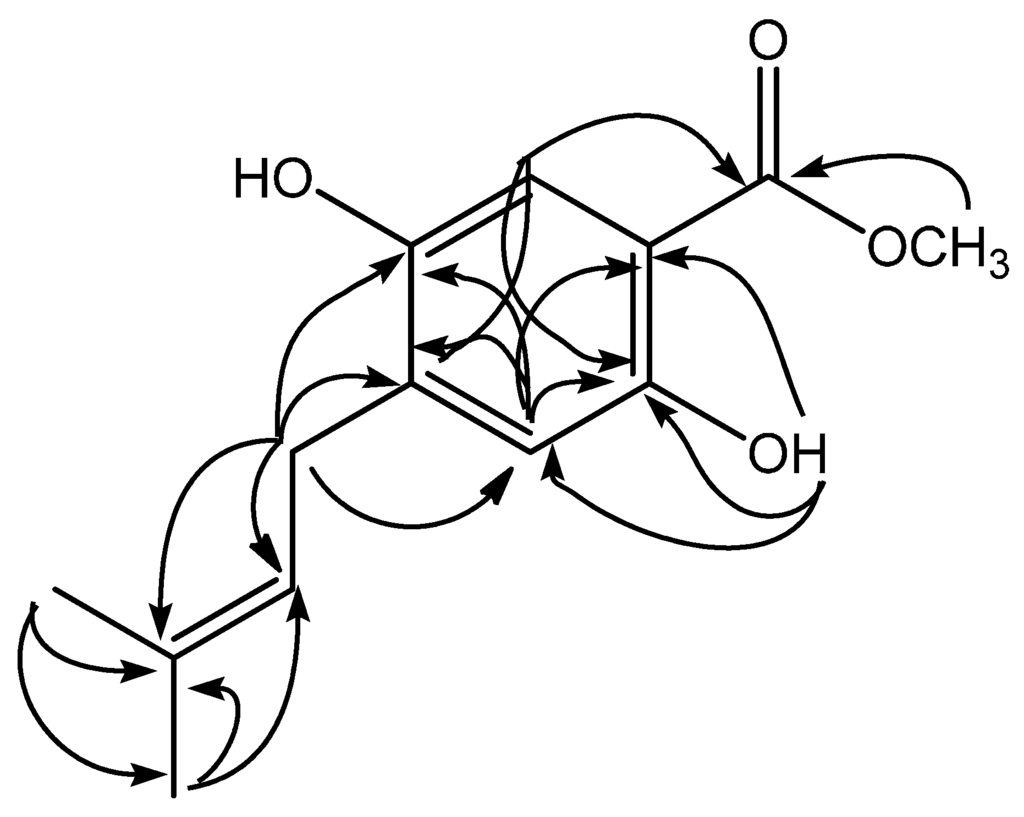
Figure 2.
Selected HMBC correlations for compound 1.
On antioxidant evaluation against DPPH radical scavenging, compound 1 exhibited IC50 values of 266.48 μg/mL. That cytotoxic data suggested that compound 1 has moderate activity.
3. Material and Methods
3.1. General Information
NMR spectra were recorded on an Agilent 500 spectrometer (Santa Clara, CA, USA) in CDCl3 at 500 (1H) and 125 (13C) MHz using TMS as the internal standard. The mass spectra was recorded using a Waters LCT Premier XE (Santa Clara, CA, USA). The UV was measured with a Shimadzu 1800 spectrophotometer (Kyoto, Japan). Vacuum-liquid chromatography (VLC) and radial chromatography were carried out using Si gel 60 GF254 and Si gel 60 PF254, for TLC analysis, and pre-coated silica gel plates (Merck, Darmstadt, Germany, Kieselgel 60 GF 254, 0,25 mm thickness) were used.
3.2. Plant Material
Samples of roots of E. subumbrans were collected in December 2014 from Botanical Garden, Pasuruan, Indonesia, and the specimen was deposited at the herbarium. The roots were cleaned, air dried under the shade, cut into small pieces and milled.
3.3. Extraction and Isolation
The dried roots of E. subumbrans (1.5 kg) were macerated in methanol at room temperature twice, and the methanol extract was evaporated under reduced pressure to give a dark brown residue (90 g). The crude extract in methanol (90 g) was partitioned first with n-hexane. The methanol layer was added with water (5% v/v) to increase the polarity and then partitioned with ethyl acetate. The ethyl acetate extract (18 g) was separated by vacuum liquid chromatography on silica gel. Elution with n-hexane–ethyl acetate by gradient amount of ethyl acetate (90:10, 80:20; 70:30; 50:50 and 20:80) to give five major fractions A–E. TLC on fraction B (460 mg) using eluent n-hexane–chloroform 1:1, showed two major spots. Purification of this fraction using planar radial chromatography, and eluting with n hexane–chloroform (from 2:8 to 1:1) yielded compound 1 (48 mg).
3.4. DPPH Radical Scavenging
The antioxidant assay of compound 1 against DPPH (2,2-diphenyl-1-picrihidrazil) radical was measured by UV spectrometer at λ 517 nm as described previously [9,10]. The inhibition percentage (%) of radical scavenging activity was calculated using the following equation:
where Ao is the absorbance of the control reaction (containing all reagents except the test compound), and As is the absorbance of the test compound.
Inhibition (%) = (Ao − As/Ao) × 100
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 41H-NMR, 13C-NMR and HRESIMS spectra are reported in the supplementary materials as Figure S1–S5 together with structure refinement parameters as Table S1. They and the molfiles can be found at http://www.mdpi.com/1422-8599/2016/2/M892.
Acknowledgments
This research was supported by Directorate General of Higher Education, Ministry of Education and Culture, Republic of Indonesia (Penelitian Hibah Fakultas, Universitas Airlangga, 2013).
Author Contributions
Mulyadi Tanjung designed the whole experiment and contributed to the manuscript. Tjitjik Srie Tjahjandarie researched data and wrote the manuscript, Ratih Dewi Saputri analyzed the NMR and HRESIMS spectra. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest
References
- Ozawa, M.; Kawamata, S.; Etoh, T.; Hayashi, M.; Komiyama, K.; Kishida, A.; Kuroda, C.; Ohsaki, A. Structures of new erythrinan alkaloids and nitric oxide production inhibitors from Erythrina crista-galli. Chem. Pharm. Bull. 2010, 58, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Watjen, W.; Suckow, A.K.; Rohrig, R.; Kulawik, A.; Wright, C.W.; Passreitrer, C.M. Prenylated flavonoid derivatives from the bark of Erythrina addisoniae. J. Nat. Prod. 2008, 71, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Yenesew, A.; Midiwo, J.O.; Heydenreich, M.; Schanzenbach, D.; Peter, M.G. Two isoflavanones from the stem bark of Erythrina sacleuxii. Phytochemistry 2000, 55, 457–459. [Google Scholar] [CrossRef]
- Innok, P.; Rukachaisirikul, T.; Phongpaichit, S.; Suksamrarn, A. Fuscacarpans A-C, new pterocarpans from the stems of Erytrina fusca. Fitoterapia 2010, 81, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Le, T.V.T.; Thuong, P.T.; Dao, T.T.; Ndinteh, D.T.; Mbafor, J.T.; Kang, K.W.; Oh, W.K. Cytotoxic and PTP1B inhibitory activities from Erythrina abyssinica. Bioorg. Med. Chem. Lett. 2009, 18, 6745–6749. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Na, M.K.; Dao, T.T.; Ndinteh, D.T.; Mbafor, J.T.; Park, J.; Cheong, H.; Oh, W.K. New stilbenoid with inhibitory activity on viral neuraminidases from Erythrina addisoniae. Bioorg. Med. Chem. Lett. 2010, 20, 6430–6434. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Gorzalczany, S.; Martino, V.; Acevedo, C.; Sterner, O.; Anke, T. Metabolites from endophytes of the medicinal plant Erytrina crista-galli. Z. Naturforsch. C 2005, 60, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, P.; Crombie, L.; Harper, M.F.; Rossiter, J.T.; Sanders, M.; Whiting, D.A. Mechanism and stereochemistry of the enzyme-catalysed formation of a 2,2-dimethylchromene ring from a prenylated phenol: conversion of rot-2′-enonic acid into deguelin by deguelin cyclase. J. Chem. Soc. Perkin Trans 1 1992, 13, 1685–1697. [Google Scholar] [CrossRef]
- Tanjung, M.; Tjahjandarie, T.S.; Sentosa, M.H. Antioxidant and cytotoxic agent from the rhizomes of Kaempferia pandurata. Asian Pac. J. Tropical Dis. 2013, 3, 401–404. [Google Scholar] [CrossRef]
- Tanjung, M.; Saputri, R.D.; Tjahjandarie, T.S. Antioxidant activity of two isomeric benzoxepin derivatives from the stem bark of Bauhinia acuelata L. J. Chem. Pharm. Res. 2014, 6, 705–708. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).