N′-(1,3-Dithiolan-2-ylidene)-3-(phenylamino)propanehydrazide
Abstract
:1. Introduction
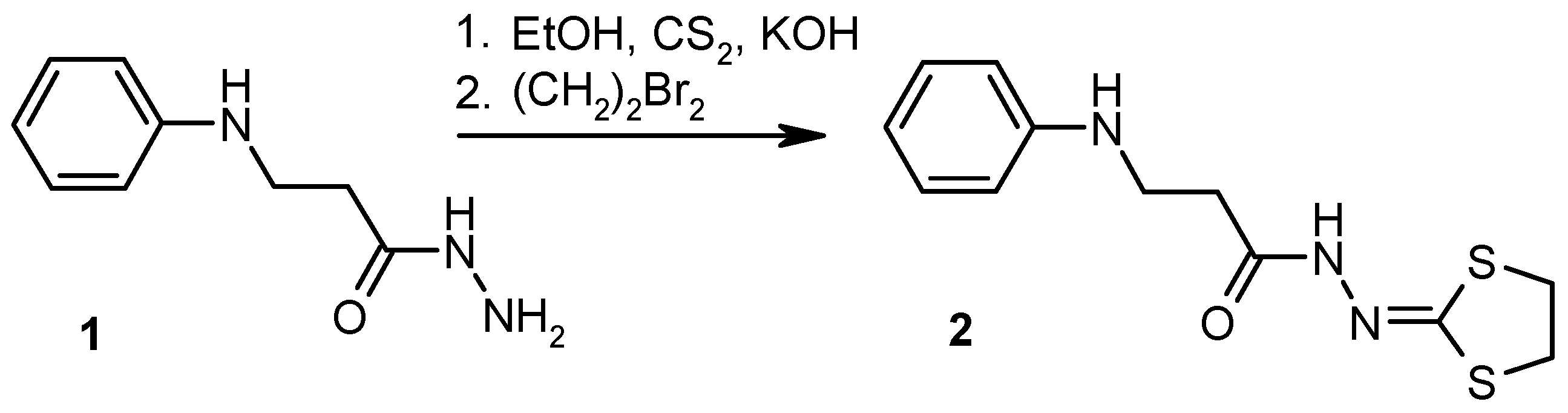
2. Results and Discussion
3. Experimental Section
3.1. General
3.2. Experimental Procedure for the Preparation of N′-(1,3-Dithiolan-2-ylidene)-3-(phenylamino)propanehydrazide (2)
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Author Contributions
Conflicts of Interest
References
- Rollas, S.; Kucukguzel, S.G. Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Hirano, A.; Takenaka, S. Selective immobilization of double stranded DNA on a gold surface through threading intercalation of a naphthalene diimide having dithiolane moieties. Anal. Chim. Acta 2010, 665, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Alfaro, M.C.; Hernandez, N.; Cerna, I.; Lopez-Cortes, J.G.; Gomez, E.; Toscano, R.A.; Alvarez-Toledano, C. Novel dinuclear iron(0) complexes from α,β-unsaturated ketones β-positioned with sulfide and sulfoxide groups. J. Organomet. Chem. 2004, 689, 885–893. [Google Scholar] [CrossRef]
- Toussaint, C.; Beghidja, C.; Welter, R. Cobalt complexes supported by salicylichydrazono derivative ligands and various coordination solvents. C. R. Chim. 2010, 13, 343–352. [Google Scholar] [CrossRef]
- Bouchameni, C.; Beghidja, C.; Beghidja, A.; Rabu, P.; Welter, R. Synthesis, structural characterizations, spectroscopic and magnetic properties of MnII and MnIII complexes with an unprecedented bridging coordination mode of 2-salicylichydrazono-1,3-dithiolane ligand. Polyhedron 2011, 30, 1774–1778. [Google Scholar] [CrossRef]
- Liang, F.; Li, Y.; Li, D.; Cheng, X.; Liu, Q. A tandem reaction of 2-acetylmethylene-1,3-dithiolanes via fragmentation of the dithiolane ring in the presence of amines: a facile route to functionalized thioamides. Tetrahedron Lett. 2007, 48, 7938–7941. [Google Scholar] [CrossRef]
- Halimehjani, A.Z.; Maleki, H.; Saidi, M.R. Regiospecific iodocyclization of S-allyl dithiocarbamates: synthesis of 2-imino-1,3-dithiolane and 2-iminium-1,3-dithiolane derivatives. Tetrahedron Lett. 2009, 50, 2747–2749. [Google Scholar] [CrossRef]
- Foks, H.; Mieczkowska, J.; Janowiec, M.; Zwolska, Z.; Andrzejczyk, Z. Synthesis and tuberculostatic activity of methyl 3-isonicotinoyldithiocarbazate and S,S'-dimethyl dithiocarbonate isonicotinoylhydrazone, and their reactions with amines and hydrazines. Chem. Heterocycl. Compd. 2002, 38, 810–816. [Google Scholar] [CrossRef]
- Bouslimani, N.; Clement, N.; Rogez, G.; Turek, P.; Choua, S.; Dagorne, S.; Welter, R. Stability, molecular structures and magnetic properties of dinuclear iron complexes supported by benzoic hydrazide derivative ligands. Inorg. Chim. Acta 2009, 363, 213–220. [Google Scholar] [CrossRef]
- Aelony, D. Storage stable epoxy B-stage resins. J. Appl. Polym. Sci. 1969, 13, 227–232. [Google Scholar] [CrossRef]
- Hesse, M.; Meier, H.; Zeeh, B. Spectroscopic Methods in Organic Chemistry, 2nd ed.; Thieme: Stuttgart, Germany, 1997; p. 468. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumosienė, I.; Kantminienė, K.; Beresnevičius, Z.J. N′-(1,3-Dithiolan-2-ylidene)-3-(phenylamino)propanehydrazide. Molbank 2015, 2015, M877. https://doi.org/10.3390/M877
Tumosienė I, Kantminienė K, Beresnevičius ZJ. N′-(1,3-Dithiolan-2-ylidene)-3-(phenylamino)propanehydrazide. Molbank. 2015; 2015(4):M877. https://doi.org/10.3390/M877
Chicago/Turabian StyleTumosienė, Ingrida, Kristina Kantminienė, and Zigmuntas J. Beresnevičius. 2015. "N′-(1,3-Dithiolan-2-ylidene)-3-(phenylamino)propanehydrazide" Molbank 2015, no. 4: M877. https://doi.org/10.3390/M877
APA StyleTumosienė, I., Kantminienė, K., & Beresnevičius, Z. J. (2015). N′-(1,3-Dithiolan-2-ylidene)-3-(phenylamino)propanehydrazide. Molbank, 2015(4), M877. https://doi.org/10.3390/M877




