Abstract
The title compound 1,4-di(2-butoxycarbonyl-trans-vinyl)-2,5-dimethoxybenzene was synthesized in 94% yield through the Heck reaction between 2,5-diiodo-1,4-dimethoxybenzene and n-butyl acrylate, using bis(dibenzylideneacetone) palladium(0) as homogeneous catalyst.
1. Introduction
Divinylbenzene and its derivatives are a very interesting class of monomer templates for the preparation of oligomeric and polymeric materials with a wide range of technological possibilities. These useful polymers have many successful and promising applications in the production of copolymers [1,2,3], resins [4,5,6], membranes [7], electroluminescent devices [8,9], fluorescent sensors [10], fluorophores [11,12], dendrimers [13,14] and helicenes [15].
p-Divinylbenzene compounds can be synthesized through different synthetic methodologies that include the Knoevenagel reaction [16,17,18], Wittig reaction [19], Heck reaction [20,21,22], Horner-Wadsworth-Emmons reaction [23], olefination of aldehydes [24,25] and others [26,27]. Unfortunately not all of these reactions provide an efficient method for the formation of pure trans isomers, which are the desired products for several applications. Among these remarkable reactions that provided pure trans products, the Heck reaction additionally allows the facile construction of structures with numerous substituents on the aromatic ring due to its compatibility with most functional groups. Specifically, in this work we describe the synthesis of 1,4-di(2-butoxycarbonyl-trans-vinyl)-2,5-dimethoxybenzene, a novel para-divinylbenzene derivative using the Heck reaction.
2. Results and Discussion
For the preparation of 3, the aryl dihalide 2 [28] was reacted with two equivalents of n-butyl acrylate 1 in dioxane under solvothermal conditions (Scheme 1). Bis(dibenzylideneacetone)palladium(0) (Pd(dba)2), triphenyl phosphite and triethylamine were used as catalyst, ligand and base respectively. The reaction was monitored using thin layer chromatography. After the purification, the desired title compound 3 was isolated in 94% yield.
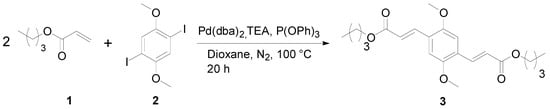
Scheme 1.
Synthesis of 1,4-di(2-butoxycarbonyl-trans-vinyl)-2,5-dimethoxybenzene 3.
The title compound was characterized by IR, 1H-NMR, 13C-NMR and elemental analysis. As expected, the IR spectrum shows a strong absorption band at 1695 cm−1 for the C=O stretching vibration. The proton NMR spectrum showed the following signals: a triplet at 0.96 ppm assigned to the CH3 groups, two multiplets at 1.43 and 1.69 ppm assigned to four CH2 groups, a singlet at 3.87 ppm assigned to OCH3 protons, a triplet at 4.21 ppm assigned to OCH2- groups, two doblets at 6.54 and 7.94 ppm corresponding to the vinyl protons with a coupling constant of 16.1 and 16.0 Hz respectively which confirms the trans configuration for the double bond, and a singlet at 7.02 ppm for the aromatic protons.
3. Experimental
3.1. General Information
Melting points, reported without correction, were measured using a Stuart SMP10 apparatus (Staffordshire, UK). The FT-IR spectra were obtained with a Shimadzu IR prestige 21 spectrophotometer (Columbia, MD, USA). 1H and 13C NMR spectra were recorded with a Bruker AVANCE III system (Billerica, MA, USA) operating at 300 MHz, using residual (δH 7.26) and deuterated solvent (δC 77.0) peaks of CDCl3 as reference standards. The elemental analysis was performed on a Thermo Scientific Flash 2000 CHNS/O analyzer (Waltham, MA, USA).
3.2. 1,4-Di(2-butoxycarbonyl-trans-vinyl)-2,5-dimethoxybenzene
A mixture of n-butyl acrylate 1 (262 μL, 3.22 mmol), 2,5-diiodo-1,4-dimethoxibenzene 2 (599.2 mg, 1.536 mmol) [28], Pd(dba)2 (8.8 mg, 0.015 mmol), triphenyl phosphite (20 μL, 0.077 mmol) and triethylamine (471 μL, 3.38 mmol) in 2 mL of dioxane was placed in a 10 mL glass vial. The vial was sealed, purged with nitrogen and stirred at 100 °C for 20 h. After cooling the mixture, 5 mL of water was added and the product was recovered by filtration. The solid was dissolved in CH2Cl2, eluted through celite to eliminate the remnant catalyst, and after evaporation of the solvent the product was finally purified by recrystallization from ethanol. The target molecule 3 (566.2 mg, 94%) was recovered as yellow crystals, m.p: 124–125 °C.
FT-IR (ATR): 2960, 2929, 1695, 1626, 1501, 1462, 1414, 1261, 1217, 1169, 1043 cm−1.
1H-NMR (300 MHz, CDCl3) δ(ppm): 0.96 (t, J = 7.4 Hz, 6H, 2CH3), 1.43 (m, 4H, 2CH2), 1.69 (m, 4H, 2CH2), 3.87 (s, 6H, 2OCH3), 4.21 (t, J = 6.7 Hz, 4H, 2OCH2), 6.54 (d, J = 16.1 Hz, 2H, =CHCO2Bu), 7.02 (s, 2H, H-Ar), 7.94 (d, J = 16.0 Hz, 2H, ArCH=).
13C-NMR (300 MHz, CDCl3) δ(ppm): 13.8 (2CH3), 19.2 (2CH2), 30.8 (2CH2), 56.0 (2OCH3), 64.5 (2OCH2), 111.1 (C(-H) ring), 119.9 (=CHCO2Bu), 126.0 (C(-C) ring), 139.0 (ArCH=), 152.5 (C(-O) ring), 167.3 (C=O).
Anal. calcd for C22H30O6: C, 67.67; H, 7.74. Found: C, 68.35; H, 7.96.
Copies of the IR, 1H, 13C-NMR spectra for compound 3 are available in the supplementary information.
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Authors wish to thank the Universidad Nacional de Colombia for financial support.
Author Contributions
The authors WM, CS, CO-P designed, accomplished research and wrote the paper together. Finally, all authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jin, J.M.; Yang, S.; Shim, S.E.; Choe, S. Synthesis of poly(acrylamide-co-divinylbenzene) microspheres by precipitation polymerization. J. Polym. Sci. Part A: Polym. Chem. 2005, 43, 5343–5346. [Google Scholar] [CrossRef]
- Donescu, D.; Raditoiu, V.; Spataru, C.I.; Somoghi, S.; Ghiurea, M.; Radovici, C.; Fierascu, R.C.; Schinteie, G.; Leca, A.; Kuncser, V. Superparamagnetic magnetite–divinylbenzene–maleic anhydride copolymer nanocomposites obtained by dispersion polymerization. Eur. Polym. J. 2012, 48, 1709–1716. [Google Scholar] [CrossRef]
- Weber, V.; Linsberger, I.; Hauner, M.; Leistner, A.; Leistner, A.; Falkenhagen, D. Neutral Styrene Divinylbenzene Copolymers for Adsorption of Toxins in Liver Failure. Biomacromolecules 2008, 9, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yu, Y.; Nguyen, S.T.; Hupp, J.T.; Broadbelt, L.J.; Farha, O.K. Epoxidation of the commercially relevant divinylbenzene with [tetrakis-(pentafluorophenyl)porphyrinato]iron(III) chloride and its derivatives. Ind. Eng. Chem. Res. 2015, 54, 922–927. [Google Scholar] [CrossRef]
- Durie, S.; Jerabek, K.; Mason, C.; Sherrington, D.C. One-pot synthesis of branched poly(styrene-divinylbenzene) suspension polymerized resins. Macromolecules 2002, 35, 9665–9672. [Google Scholar] [CrossRef]
- Chen, B.; Wang, W.; Ma, X.; Wang, C.; Li, R. Adsorption behaviors of glycerol from biodiesel on sulfonated polystyrene−divinylbenzene resins in different forms. Energ. Fuels 2012, 26, 7060–7067. [Google Scholar] [CrossRef]
- Bertran, O.; Curcó, D.; Torras, J.; Ferreira, A.; Alemán, C. Field-induced transport in sulfonated poly(styrene-co-divinylbenzene) membranes. Macromolecules 2010, 43, 10521–10527. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H. Synthesis, electrochemistry, and electroluminescence of novel red-emitting poly(p-phenylenevinylene) derivative with 2-pyran-4-ylidene-malononitrile obtained by the Heck reaction. Chem. Mater. 2002, 14, 2270–2275. [Google Scholar] [CrossRef]
- Chen, J.T.; Hsu, C.S. Poly(2,3-diphenyl-1,4-phenylenevinylene) (DP-PPV) derivatives: Synthesis, properties, and their applications in polymer light-emitting diodes. Polymer 2013, 54, 4045–4058. [Google Scholar] [CrossRef]
- Gao, W.; Yan, M.; Ge, S.; Liu, X.; Yu, J. Fluorescent sensor based on a novel conjugated polyfluorene derivative. Spectrochim. Acta Part A 2012, 95, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.T.; Son, H.S.; Ku, J.K.; Kim, B.H. Synthesis and photophysical studies of bis-enediynes as tunable fluorophores. J. Am. Chem. Soc. 2003, 125, 11241–11248. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.T.; Son, H.S.; Ku, J.K.; Kim, B.H. Novel fluorophores: Efficient synthesis and photophysical study. Org. Lett. 2001, 3, 2469–2471. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.T.; Kim, B.H. π-Conjugated dendrimers based on bis(enediynyl)benzene units. Org. Lett. 2004, 6, 2669–2672. [Google Scholar] [CrossRef] [PubMed]
- Kaafarani, B.R.; Wex, B.; Wang, F.; Catanescu, O.; Chien, L.C.; Neckers, D.C. Synthesis of highly fluorescent Y-enyne dendrimers with four and six arms. J. Org. Chem. 2003, 68, 5377–5380. [Google Scholar] [CrossRef] [PubMed]
- Gingras, M. One hundred years of helicene chemistry. Part 1: Non-stereoselective syntheses of carbohelicenes. Chem. Soc. Rev. 2013, 42, 968–1006. [Google Scholar] [CrossRef] [PubMed]
- List, B.; Doehring, A.; Fonseca, m.T.H.; Wobser, K.; Van-Thienen, H.; Torres, R.R.; Galilea, P.L. Practical synthesis of (E)-α,β-unsaturated esters from aldehydes. Adv. Synth. Catal. 2005, 347, 1558–1560. [Google Scholar] [CrossRef]
- Kilway, K.V.; Siegel, J.S. Control of functional group proximity and direction by conformational networks: Synthesis and stereodynamics of persubstituted arenes. Tetrahedron 2001, 57, 3615–3627. [Google Scholar] [CrossRef]
- Irngartinger, H.; Herpich, R. Synthesis and topochemistry of 2,5-bisacrylate-substituted 1,4-benzoquinones. Eur. J. Org. Chem. 1998, 595–604. [Google Scholar] [CrossRef]
- Vatèle, J.-M. One-pot selective oxidation/olefination of primary alcohols using TEMPO–BAIB system and stabilized phosphorus ylides. Tetrahedron Lett. 2006, 47, 715–718. [Google Scholar] [CrossRef]
- Wang, A.-E.; Xie, J.-H.; Wang, L.-X.; Zhou, Q.-L. Triaryl phosphine-functionalized N-heterocyclic carbene ligands for Heck reaction. Tetrahedron 2005, 61, 259–266. [Google Scholar] [CrossRef]
- Cui, X.; Li, Z.; Tao, C.-Z.; Xu, Y.; Li, J.; Liu, L.; Guo, Q.-X. N,N-Dimethyl-β-alanine as an inexpensive and efficient ligand for palladium-catalyzed Heck reaction. Org. Lett. 2006, 8, 2467–2470. [Google Scholar] [PubMed]
- Dai, M.; Liang, B.; Wang, C.; Chen, J.; Yang, Z. Synthesis of a novel C2-symmetric thiourea and its application in the Pd-catalyzed cross-coupling reactions with arenediazonium salts under aerobic conditions. Org. Lett. 2004, 6, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Kanbara, M.; Ohashi, M.; Hayase, S.; Kawatsura, M.; Kato, T.; Miyazawa, K.; Takagi, Y.; Uno, H. gem-Difluorocyclopropane as core molecule candidate for liquid crystal compounds. J. Fluorine Chem. 2007, 128, 1112–1120. [Google Scholar] [CrossRef]
- Sun, W.; Yu, B.; Kühn, F.E. Ruthenium(II)–salen complexes-catalyzed olefination of aldehydes with ethyl diazoacetate. Tetrahedron Lett. 2006, 47, 1993–1996. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Korotchenko, V.N.; Shastin, A.V.; Balenkova, E.S. Catalytic olefination of carbonyl compounds. A new versatile method for the synthesis of alkenes. Russ. Chem. Bull. 2004, 53, 1034–1064. [Google Scholar] [CrossRef]
- Oh, C.H.; Lim, Y.M.; You, C.H. Platinum-catalyzed cross-couplings of organoboronic acids with aryl iodides. Tetrahedron Lett. 2002, 43, 4645–4647. [Google Scholar] [CrossRef]
- Katayama, H.; Nagao, M.; Nishimura, T.; Matsui, K.; Umeda, K.; Akamatsu, K.; Tsuruoka, T.; Nawafune, H.; Ozawa, F. Stereocontrolled synthesis and optical properties of all-cis poly(phenylene vinylenes) (PPVs): A method for direct patterning of PPVs. J. Am. Chem. Soc. 2005, 127, 4350–4353. [Google Scholar] [CrossRef] [PubMed]
- Sierra, C.A.; Lahti, P.M. A photoluminescent, segmented oligo-polyphenylenevinylene copolymer with hydrogen-bonding pendant chains. Chem. Mater. 2004, 16, 55–61. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).