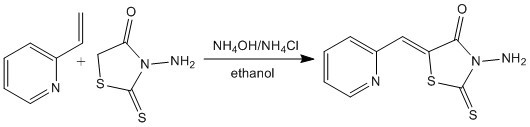
(Z)-3-Amino-5-(pyridin-2-ylmethylidene)-2-thioxo-1,3-thiazolidin-4-one
Abstract
:1. Introduction
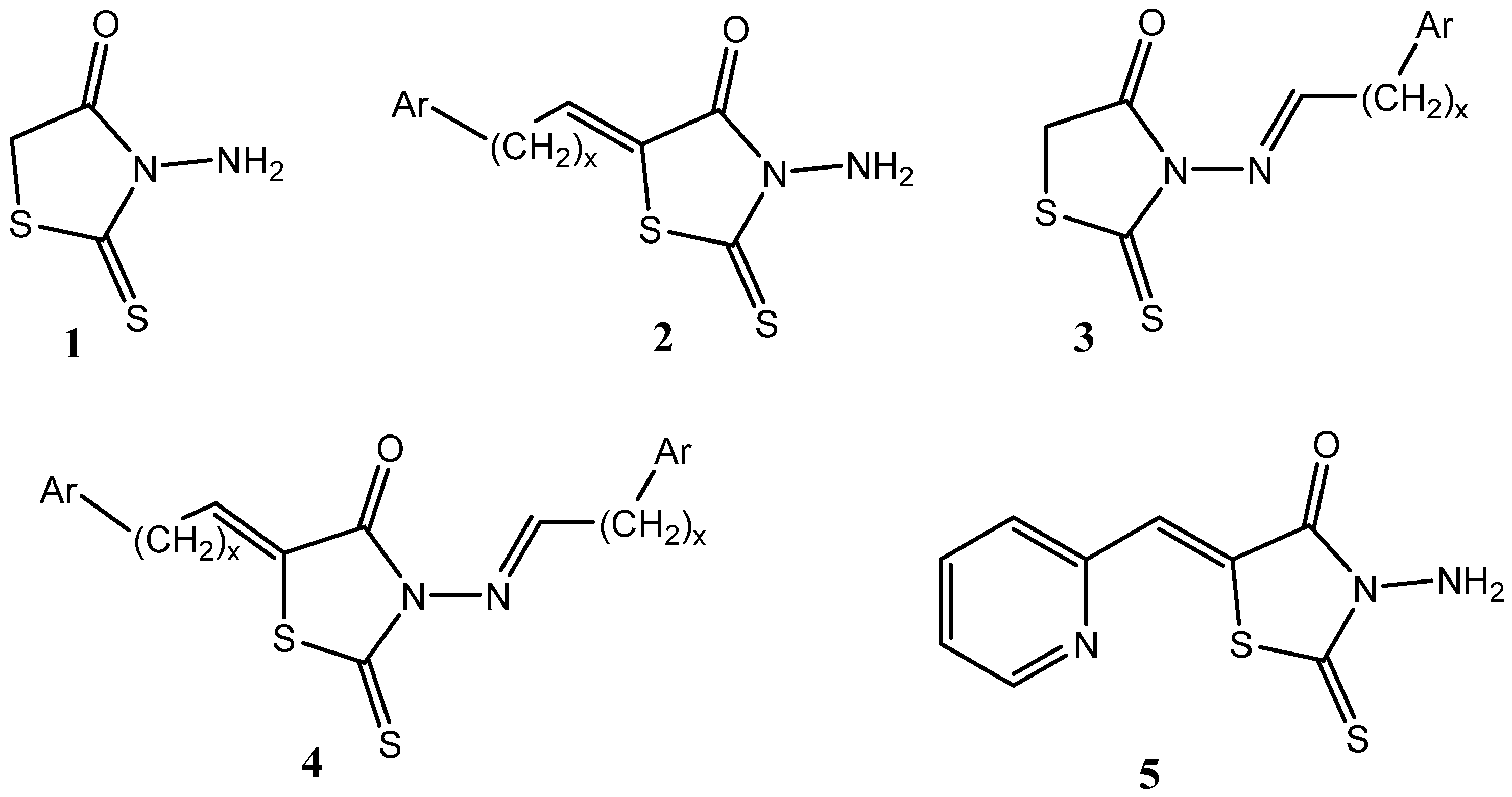
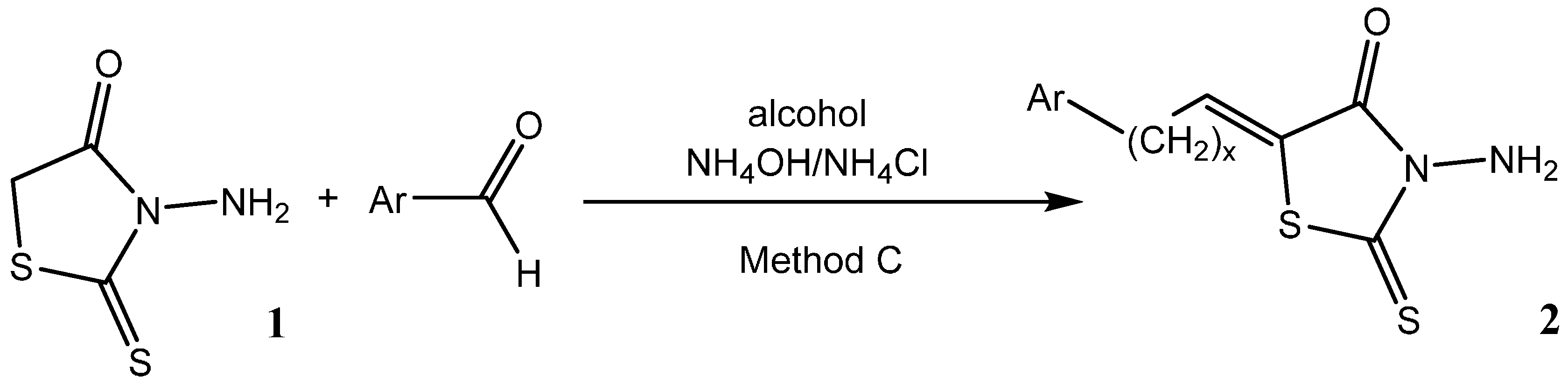
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Experimental Procedure for the Preparation of 3-Amino-5-(pyridin-2-ylmethylidene)-2-thioxo-1,3-thiazolidin-4-one (5)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tomasic, T.; Masic, L.P. Rhodanine as a privileged scaffold in drug discovery. Curr. Med. Chem. 2009, 16, 1596–1629. [Google Scholar] [CrossRef] [PubMed]
- Tomasic, T.; Peterlin Masic, L. Rhodanine as a scaffold in drug discovery: A critical review of its biological activities and mechanisms of target modulation. Expert Opin. Drug Discov. 2012, 7, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Dolezel, J.; Opletalova, V.; Vejsova, M.; Hirsova, P. Biological Evaluation of Thiazolidine Derivatives. Abstracts of the 5th International Postgraduate Research Symposium on Pharmaceutics (IPORSIP-2007), Istanbul, Turkey, 13–15 September 2007. Acta Pharm. Sci. 2007, 49 (Suppl.), 31. [Google Scholar]
- Petrik, P.; Kunes, J.; Vejsova, M.; Jampilek, J.; Spaningerova, E.; Kesetovicova, D.; Vlckova, M.; Majd, M.; Kalafutova, S.; Opletalova, V. Antifungal and antimycobacterial properties of 5-arylmethylidenerhodanines and their N3-substituted analogues. Abstracts of the 5th International Postgraduate Research Symposium on Pharmaceutics (IPORSIP-2007), Istanbul, Turkey, 13–15 September 2007. Acta Pharm. Sci. 2007, 49 (Suppl.), 77. [Google Scholar]
- Dolezel, J.; Hirsova, P.; Opletalova, V.; Dohnal, J.; Vejsova, M.; Kunes, J.; Jampilek, J. Rhodanineacetic acid derivatives as potential drugs: Preparation, hydrophobic properties and antifungal activity of (5-arylalkylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)acetic acids. Molecules 2009, 14, 4197–4212. [Google Scholar] [CrossRef] [PubMed]
- Opletalova, V.; Dolezel, J.; Buchta, V.; Vejsova, M.; Paterova, P. Antifungal effects of (5Z)-5-arylmethylidenerhodanines with a special view to members of Mucorales. Folia Pharm. Univ. Carol. 2014, 42, 7–13. [Google Scholar]
- Opletalova, V.; Dolezel, J.; Kralova, K.; Pesko, M.; Kunes, J.; Jampilek, J. Synthesis and characterization of (Z)-5-arylmethylidenerhodanines with photosynthesis-inhibiting properties. Molecules 2011, 16, 5207–5227. [Google Scholar] [CrossRef] [PubMed]
- Andreasch, R. Substituted rhodaninic acids and their aldehyde condensation products (VII). Monatsh. Chem. 1908, 29, 399–419. [Google Scholar] [CrossRef]
- Stainier, C.; Lapiere, C.L. Some derivatives of rhodanine with in vitro tuberculostatic action. Bull. Acad. R. Med. Belg. 1958, 23, 335–345. [Google Scholar] [PubMed]
- Lapiere, C.L. Aminorhodanine derivatives: Syntheses and tuberculostatic action. J. Pharm. Belg. 1959, 14, 126–140. [Google Scholar] [PubMed]
- Petlichnaya, L.I.; Turkevich, M.M. Some properties of 3-aminorhodanine. Dopov. Akad. Nauk Ukr. RSR 1965, 1601–1603, Chem. Abstr. 1966, 64, 59854. [Google Scholar]
- Petlichnaya, L.I. Double reactivity of 3-aminorhodanines. Khim. Geterotsikl. Soedin. 1967, 3, 649–652, Chem. Abstr. 1968, 68, 78182. [Google Scholar]
- Petlichnaya, L.I.; Turkevich, N.M. Synthesis of the new arylidene derivatives of 3-aminorhodanine. Khim. Geterotsikl. Soedin. 1968, 4, 73–75, Chem. Abstr. 1968, 69, 77148. [Google Scholar] [CrossRef]
- Turkevich, N.M.; Kontsevich, L.S.; Petlichnaya, L.I. 3-Aminorhodanine and its derivatives. Zb. Nauk Prats. L’vivsk. Med. Inst. 1963, 24, 22–28, Chem. Abstr. 1965, 62, 51596. [Google Scholar]
- Turkevich, M.M.; Tarasevicius, E. Synthesis of 5-arylidene derivatives of 3-(5-nitrofurfurylidene)aminorhodanine. Farm. Zh. (Kiev) 1968, 23, 44–47, Chem. Abstr. 1968, 69, 77154. [Google Scholar]
- Turkevich, N.M.; Petlichnaya, L.I. Ultraviolet absorption spectra of 3-aminorhodanine derivatives. Khim. Geterotsikl. Soedin. 1971, 7, 1182–1185, Chem. Abstr. 1972, 76, 58466. [Google Scholar]
- Sandstrom, J. Carboxydithiocarbohydrazides and N-aminorhodanines. Ark. Kemi 1955, 8, 487–521, Chem. Abstr. 1956, 50, 64634. [Google Scholar]
- Sugihara, A.; Ito, M. Nitrofuran derivatives. II. Condensation of nitrofuryl aldehydes with amines or 2-methyl-1,3,4-oxadiazoles. Yakugaku Zasshi 1965, 85, 424–429, Chem. Abstr. 1965, 63, 31685. [Google Scholar]
- Hanefeld, W.; Schlitzer, M. Regioselective condensations and N-acylations of 3-aminorhodanines. Arch. Pharm. 1993, 326, 887–891. [Google Scholar] [CrossRef]
- Stankovic, E.; Elecko, P.; Toma, S. Knoevenagel condensation of ferrocenecarbaldehyde with some methylene active reagents on inorganic supports. Chem. Pap. 1996, 50, 68–71. [Google Scholar]
- Krutosikova, A.; Lacova, M.; Dandarova, M.; Chovancova, J. Effect of microwave irradiation on reaction of furo[3,2-b]pyrrole- and furo[2,3-b]pyrrole-2-carbaldehydes with some active methylene compounds. ARKIVOC 2000, iii, 409–420. [Google Scholar]
- Afkhami, A.; Madrakian, T.; Ahmadi, R.; Bagheri, H.; Tabatabaee, M. Chemically modified alumina nanoparticles for selective solid phase extraction and preconcentration of trace amounts of Cd(II). Microchim. Acta 2011, 175, 69–77. [Google Scholar] [CrossRef]
- Tabatabaee, M.; Heravi, M.M.; Sharif, M.; Esfandiyari, F. Fast and efficient method for imination of N-aminorhodanine using inorganic solid support under microwave irradiation and classical heating. E-J. Chem. 2011, 8, 535–540. [Google Scholar] [CrossRef]
- Shivhare, S.; Mishra, R.; Gharia, A.; Gautam, M.D. Synthesis, characterization and antimicrobial studies of Cu(II), Co(II) and Ni(II) complexes with Schiff base derived from N-aminorhodanine and salicylaldehyde. Int. J. Pharm. Pharm. Sci. 2012, 4 (Suppl. 1), 394–397. [Google Scholar]
- Guiheneuf, S.; Paquin, L.; Carreaux, F.; Durieu, E.; Roisnel, T.; Meijer, L.; Bazureau, J.-P. New 5-ylidene rhodanine derivatives based on the dispacamide a model. Mol. Divers. 2014, 18, 375–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, L.T.A.; Nguyen, T.C.; Tai, B.H.; Pham, H.Y.; Nguyen, X.N.; Thao, D.T.; Nguyen, H.N.; Minh, C.V.; Kiem, P.V.; Kim, Y.H. Synthesis of chromonylthiazolidines and their cytotoxicity to human cancer cell lines. Molecules 2015, 20, 1151–1160. [Google Scholar]
- Khodair, A.I. A convenient synthesis of 2-arylidene-5H-thiazolo[2,3-b]quinazoline-3,5[2H]-diones and their benzoquinazoline derivatives. J. Heterocycl. Chem. 2002, 39, 1153–1160. [Google Scholar] [CrossRef]
- Ohishi, Y.; Mukai, T.; Nagahara, M.; Yajima, M.; Kajikawa, N.; Miyahara, K.; Takano, T. Preparations of 5-alkylmethylidene-3-carboxymethylrhodanine derivatives and their aldose reductase inhibitory activity. Chem. Pharm. Bull. 1990, 38, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Zidar, N.; Tomasic, T.; Sink, R.; Rupnik, V.; Kovac, A.; Turk, S.; Patin, D.; Blanot, D.; Contreras Martel, C.; Dessen, A.; et al. Discovery of novel 5-benzylidenerhodanine and 5-benzylidenethiazolidine-2,4-dione inhibitors of MurD ligase. J. Med. Chem. 2010, 53, 6584–6594. [Google Scholar] [CrossRef] [PubMed]
- Tomasic, T.; Zidar, N.; Mueller-Premru, M.; Kikelj, D.; Masic, L.P. Synthesis and antibacterial activity of 5-ylidenethiazolidin-4-ones and 5-benzylidene-4,6-pyrimidinediones. Eur. J. Med. Chem. 2010, 45, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Tomasic, T.; Kovac, A.; Simcic, M.; Blanot, D.; Golic Grdadolnik, S.; Gobec, S.; Kikelj, D.; Peterlin Masic, L. Novel 2-thioxothiazolidin-4-one inhibitors of bacterial MurD ligase targeting d-Glu- and diphosphate-binding sites. Eur. J. Med. Chem. 2011, 46, 3964–3975. [Google Scholar] [CrossRef] [PubMed]
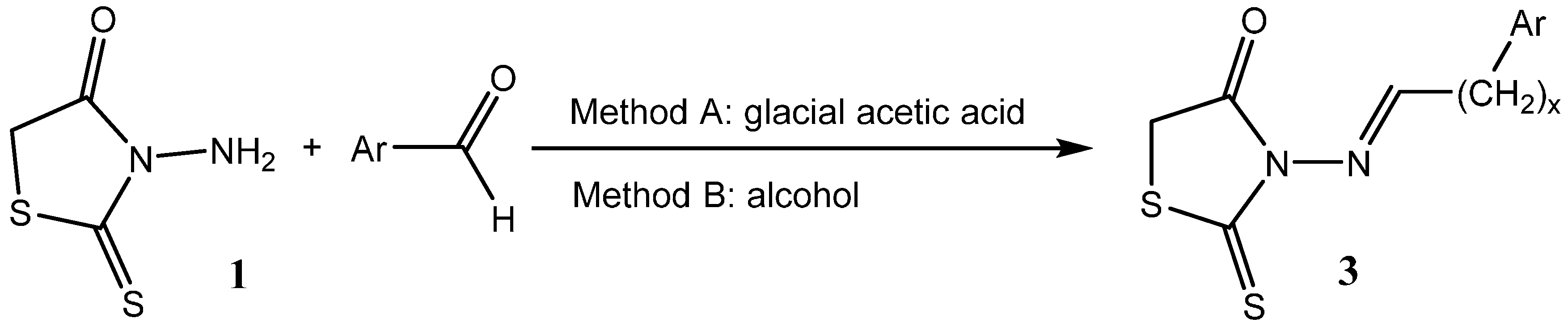
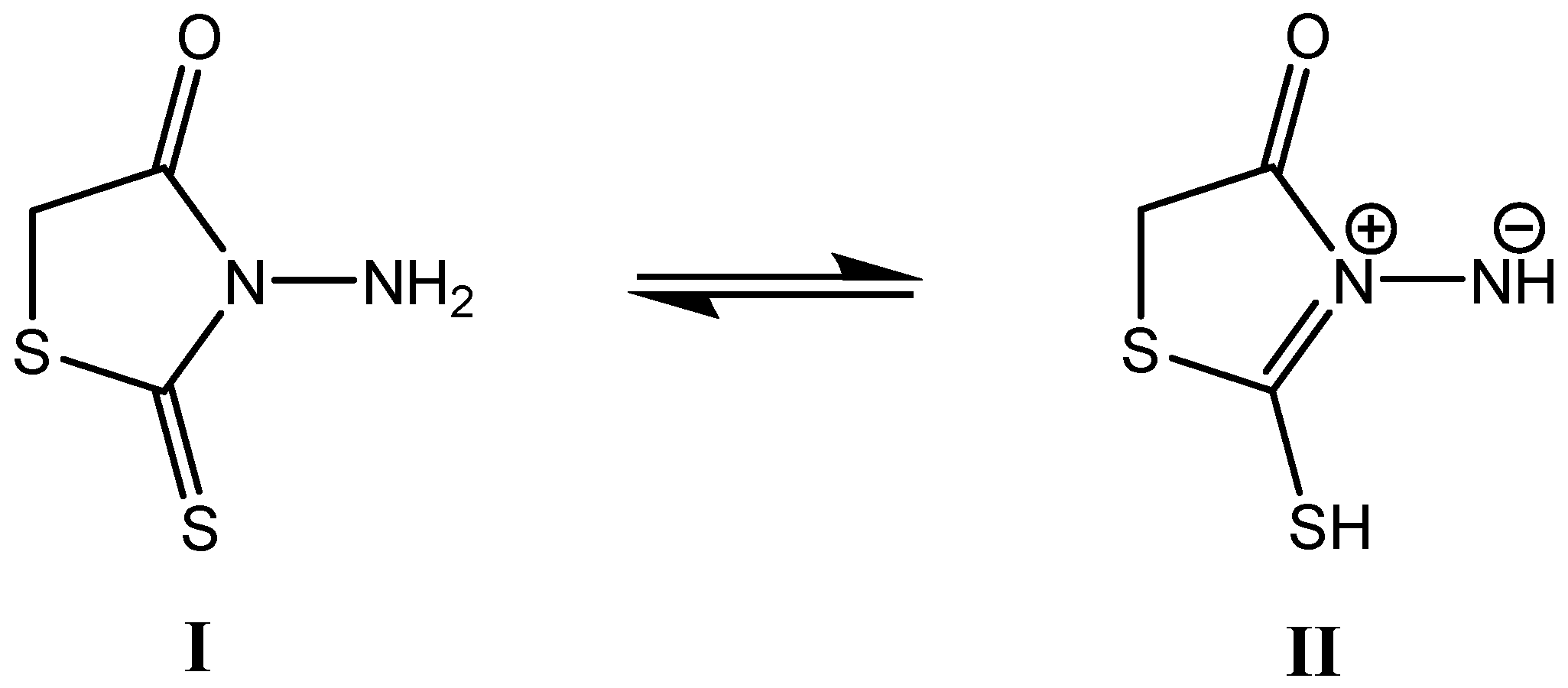
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirsova, P.; Dolezel, J.; Kucerova-Chlupacova, M.; Kunes, J.; Pilarova, V.; Novakova, L.; Opletalova, V. (Z)-3-Amino-5-(pyridin-2-ylmethylidene)-2-thioxo-1,3-thiazolidin-4-one. Molbank 2015, 2015, M872. https://doi.org/10.3390/M872
Hirsova P, Dolezel J, Kucerova-Chlupacova M, Kunes J, Pilarova V, Novakova L, Opletalova V. (Z)-3-Amino-5-(pyridin-2-ylmethylidene)-2-thioxo-1,3-thiazolidin-4-one. Molbank. 2015; 2015(4):M872. https://doi.org/10.3390/M872
Chicago/Turabian StyleHirsova, Petra, Jan Dolezel, Marta Kucerova-Chlupacova, Jiri Kunes, Veronika Pilarova, Lucie Novakova, and Veronika Opletalova. 2015. "(Z)-3-Amino-5-(pyridin-2-ylmethylidene)-2-thioxo-1,3-thiazolidin-4-one" Molbank 2015, no. 4: M872. https://doi.org/10.3390/M872
APA StyleHirsova, P., Dolezel, J., Kucerova-Chlupacova, M., Kunes, J., Pilarova, V., Novakova, L., & Opletalova, V. (2015). (Z)-3-Amino-5-(pyridin-2-ylmethylidene)-2-thioxo-1,3-thiazolidin-4-one. Molbank, 2015(4), M872. https://doi.org/10.3390/M872