Methyl 3,4-Dicarboxy-3-hydroxyeicosanoate
Abstract
:Introduction
Results and Discussion
Experimental
General
Biological Material
Extraction and Isolation
Methyl Ester Derivative of Compound 1
Supplementary materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Denton-Giles, M.; Bradshaw, R.E.; Dijkwel, P.P. Ciborinia camelliae (Sclerotiniaceae) Induces Variable Plant Resistance Responses in Selected Species of Camellia. Phytopathology 2013, 103, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Otta, Y.; Amano, K.; Nishiyama, K.; Ando, A.; Ogawa, S.; Nagata, Y. Purification and Properties of a Lectin from Ascomycete Mushroom, Ciborinia camelliae. Phytochemistry 2002, 60, 103–107. [Google Scholar] [CrossRef]
- Taylor, C.H.; Long, P.G. Review of Literature on Camellia Flower Blight Caused by Ciborinia camelliae. N. Z. J. Crop Hortic. Sci. 2000, 28, 123–138. [Google Scholar] [CrossRef]
- Thoms, H.; Vogelsang, J. To the Knowledge of Agricin Acid. Justus. Liebigs Ann. Chem. 1907, 357, 145–170. [Google Scholar] [CrossRef]
- García, N.; Zazueta, C.; Pavón, N.; Chávez, E. Agaric Acid Induces Mitochondrial Permeability Transition Through Its Interaction with the Adenine Nucleotide Translocase. Its Dependence on Membrane Fluidity. Mitochondrion 2005, 5, 272–281. [Google Scholar] [CrossRef] [PubMed]
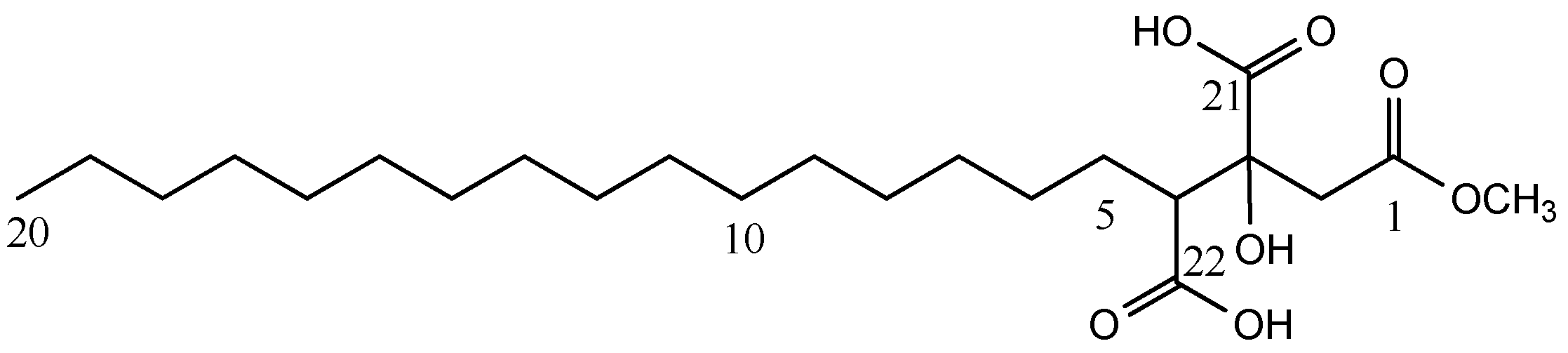
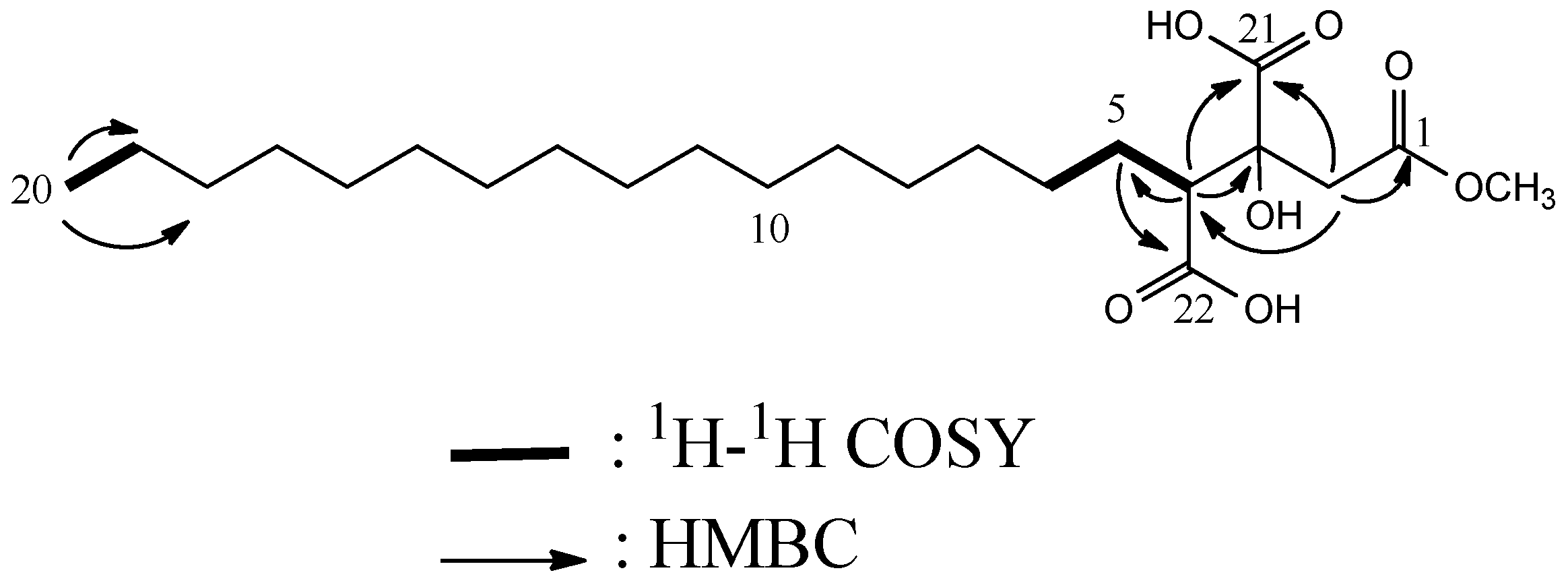
| Compound 1 | |||
|---|---|---|---|
| No. | δC, type | δH (J in Hz) | HMBC |
| 1 | 172.3 C | ||
| 2 | 23.7 CH2 | 2.72, d (15.9) | 1, 4, 21 |
| 3.10, d (15.9) | 1, 4, 21 | ||
| 3 | 76.9 C | ||
| 4 | 54.8 CH | 2.65, dd, (9.0, 2.5) | 3, 5, 21 |
| 5 | 28.2 CH2 | 1.73, m | |
| 2.21, m | 22 | ||
| 6–19 | * | 1.26–1.28, (m) | |
| 20 | 14.5 CH3 | 0.89, t (7.4) | 18, 19 |
| 21 | 176.4 a C | ||
| 22 | 176.2 a C | ||
| OMe | 52.1 CH3 | 3.64, (s) | 1 |
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiono, Y.; Murayama, T.; Koseki, T. Methyl 3,4-Dicarboxy-3-hydroxyeicosanoate. Molbank 2015, 2015, M861. https://doi.org/10.3390/M861
Shiono Y, Murayama T, Koseki T. Methyl 3,4-Dicarboxy-3-hydroxyeicosanoate. Molbank. 2015; 2015(2):M861. https://doi.org/10.3390/M861
Chicago/Turabian StyleShiono, Yoshihito, Tetsuya Murayama, and Takuya Koseki. 2015. "Methyl 3,4-Dicarboxy-3-hydroxyeicosanoate" Molbank 2015, no. 2: M861. https://doi.org/10.3390/M861
APA StyleShiono, Y., Murayama, T., & Koseki, T. (2015). Methyl 3,4-Dicarboxy-3-hydroxyeicosanoate. Molbank, 2015(2), M861. https://doi.org/10.3390/M861





