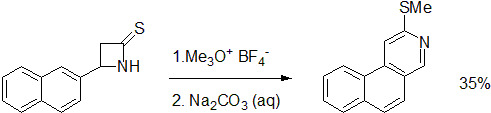2-Methylsulfanylbenzo[f]isoquinoline
Abstract
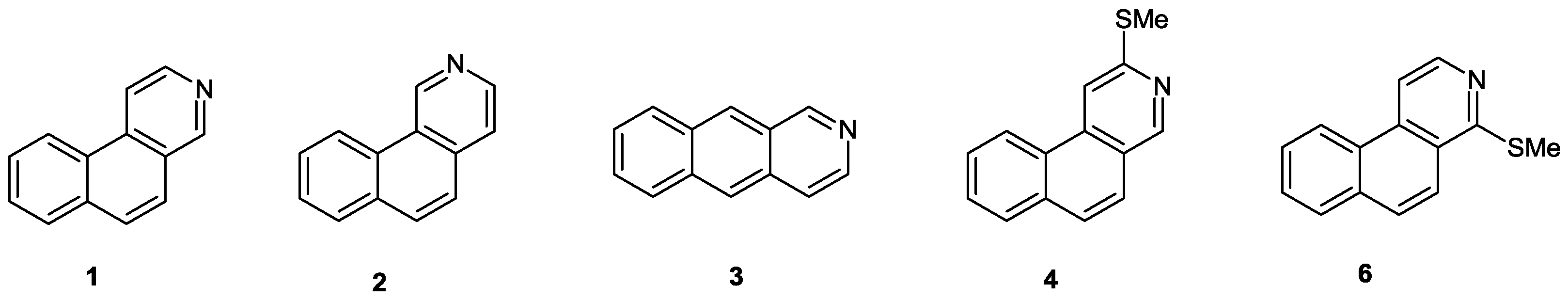
:Introduction
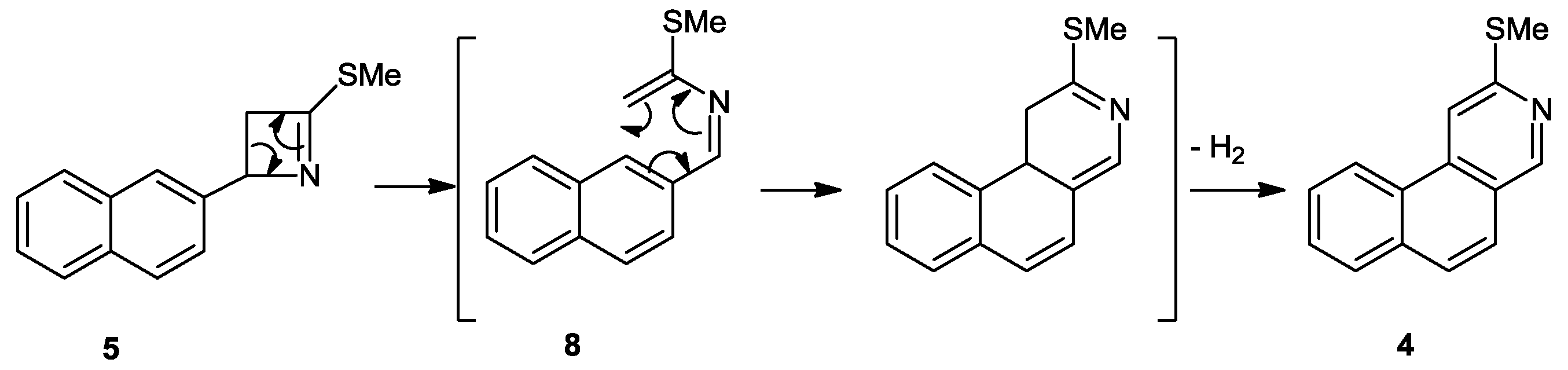
Discussion
Experimental
Spectroscopic Data
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Hoshina, H.; Kubo, K.; Morita, A.; Sakurai, T. Formation of Isoquinoline and 1-Azetine Derivatives via Novel Photocyclization of Substituted α-Dehydrophenylalanines. Tetrahedron 2000, 56, 2941–2951. [Google Scholar] [CrossRef]
- Maekawa, K.; Igarashi, T.; Kubo, K.; Sakurai, T. Electron transfer-initiated photocyclization of substituted N-acetyl-α-dehydro(1-naphthyl)alanines to 1,2-dihydrobenzo[f]quinoline derivatives: scope and limitations. Tetrahedron 2001, 57, 5515–5526. [Google Scholar] [CrossRef]
- Lalezari, I.; Nabahi, S. Polyazasteroids. III. 1,2,4-Triazolo[3,4-a]benzo[f]isoquinoline. Synthesis of the Diazaphenanthrene Alkaloid Perlolidine and its 1,8-Phenanthroline Isomer. J. Heterocycl. Chem. 1980, 17, 1761–1763. [Google Scholar] [CrossRef]
- Cappelli, A.; Anzini, M.; Vomero, S.; Canullo, L.; Mennuni, L.; Makovec, F.; Doucet, E.; Hamon, M.; Menziani, M.C.; de Benedetti, P.G.; et al. Novel Potent and Selective Central 5-HT3 Receptor Ligands Provided with Different Intrinsic Efficacy. J. Med. Chem. 1999, 42, 1556–1575. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, Y.; Hong, X.; Xu, B. Mn(II)/O2-promoted oxidative annulation of vinyl isocyanides with boronic acids: Synthesis of multi-substituted isoquinolines. Chem. Commun. 2014, 50, 13485–13488. [Google Scholar] [CrossRef] [PubMed]
- Lechel, T.; Dash, J.; Eidamshaus, C.; Brüdgam, I.; Lentz, D.; Reissig, H.-U. A three-component synthesis of β-alkoxy-β-keto-enamides—flexible precursors for 4-hydroxypyridine derivatives and their palladium-catalysed reactions. Org. Biomol. Chem. 2010, 8, 3007–3014. [Google Scholar] [CrossRef] [PubMed]
- Villuendas, P.; Urriolabeitia, E.P. Primary Amines as Directing Groups in the Ru-Catalyzed Synthesis of Isoquinolines, Benzoisoquinolines and Thienopyridines. J. Org. Chem. 2013, 78, 5254–5263. [Google Scholar] [CrossRef] [PubMed]
- Garbaccio, R.M.; Huang, S.; Tasber, E.S.; Fraley, M.E.; Yan, Y.; Munshi, S.; Ikuta, M.; Kuo, L.; Kreatsoulas, C.; Stirdivant, S.; et al. Synthesis and evaluation of substituted benzoisoquinolinones as potent inhibitors of Chk1 kinase. Bioorg. Med. Chem. Lett. 2007, 17, 6280–6285. [Google Scholar] [CrossRef] [PubMed]
- Hemming, K.; Khan, M.N.; O’Gorman, P.A.; Pitard, A. 1,2,4-Oxadiazoles from cycloreversions of oxadiazabicyclo[3.2.0]heptenes: 1-Azetines as thiocyanate equivalents. Tetrahedron 2013, 69, 1279–1284. [Google Scholar] [CrossRef]
- Hemming, K.; Khan, M.N.; Kondakal, V.V.R.; Pitard, A.; Qamar, M.I.; Rice, C.R. Pyridines from Azabicyclo[3.2.0]hept-2-en-4-ones through a Proposed Azacyclopentadienone. Org. Lett. 2012, 14, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Novikov, M.S.; Smetanin, I.A.; Khlebnikov, A.F.; Rostovskii, N.V.; Yufit, D.S. Synthesis of electron-poor 4-halo-2-azabuta-1,3-dienes by Rh(II)-catalyzed diazo ester-azirine coupling. 2-Azabuta-1,3-diene-2,3-dihydroazete valence isomerism. Tetrahedron Lett. 2012, 53, 5777–5780. [Google Scholar] [CrossRef]
- Sugie, M.; Takeo, H.; Matsumura, C. A Study of the Thermal Decomposition and Dehydrochlorination of N-Chloroazetidine: Microwave Spectra of N-Chloromethylenimine, 1-Azetine, and 2-Azabutadiene. J. Am. Chem. Soc. 1989, 111, 906–910. [Google Scholar] [CrossRef]
- Guillemin, J.C.; Denis, J.M.; Lablache-Combier, A. 1-Azetine: Thermal Ring Opening to 2-Azabutadiene. J. Am. Chem. Soc. 1981, 103, 468–469. [Google Scholar] [CrossRef]
- Deutsch, J.; Prescott, H.A.; Müller, D.; Kemnitz, E.; Lieske, H. Acylation of naphthalenes and anthracene on sulfated zirconia. J. Catal. 2005, 231, 269–278. [Google Scholar] [CrossRef]
- Sörgel, S.; Azap, C.; Reissig, H.-U. Preparation of Highly Substituted Naphthaldehyde Derivatives—A Regioselective Approach to Building Blocks for the Synthesis of Rubromycins. Eur. J. Org. Chem. 2006, 2006, 4405–4418. [Google Scholar] [CrossRef]
- Grigg, R.; Kongkathip, N.; Kongkathip, B.; Luangkamin, S.; Dondas, H.A. Palladium catalysed reaction of allene with phenols. Phenoxymethyl-1,3-dienes and their further reactions. Tetrahedron 2001, 57, 7965–7978. [Google Scholar] [CrossRef]

© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamshaid, F.; Khan, M.N.; Hemming, K. 2-Methylsulfanylbenzo[f]isoquinoline. Molbank 2015, 2015, M860. https://doi.org/10.3390/M860
Jamshaid F, Khan MN, Hemming K. 2-Methylsulfanylbenzo[f]isoquinoline. Molbank. 2015; 2015(2):M860. https://doi.org/10.3390/M860
Chicago/Turabian StyleJamshaid, Faisal, Musharraf N. Khan, and Karl Hemming. 2015. "2-Methylsulfanylbenzo[f]isoquinoline" Molbank 2015, no. 2: M860. https://doi.org/10.3390/M860
APA StyleJamshaid, F., Khan, M. N., & Hemming, K. (2015). 2-Methylsulfanylbenzo[f]isoquinoline. Molbank, 2015(2), M860. https://doi.org/10.3390/M860