Abstract
A novel N-benzyl-(3E,5E)-3,5-bis(2-hydroxybenzylidene)-4-piperidone (3), was synthesized in good yield by a condensation reaction of 2-hydroxybenzaldehyde (1) and N-benzyl-4-piperidone (2) under microwave irradiation in the presence of 10% NaOH solution. The chemical structure was assigned on the basis of UV-visible, IR, 1H-NMR, 13C-NMR and mass spectral data.
Curcumin (diferuloylmethane) is an orange-yellow and dietary polyphenolic phytochemical in turmeric (Curcuma longa). In recent decades, curcumin has been shown to exhibit antioxidant [1], anti-inflammatory [2], antiviral [3], antibacterial [4] effects, and thus has potential use against various malignant cancers, diabetes, allergies, arthritis and other chronic illnesses [5,6,7,8]. For the purpose of finding novel derivatives with increased systemic bioavailability and enhanced pharmacological activity [9], chemical modifications as well as synthesis of curcumin analogues have been attempted by many research groups to find a better treatment for various diseases [9,10]. Analogous compounds to (E)-3,5-bis(benzylidene)-4-piperidones presented noteworthy cytotoxic activity against leukimia cell lines and colon cancer among others [11]. Different substituents with opposing electronic properties in the benzene rings were designed to investigate and discuss the structure–activity relationship [12]. During the course of our continuing search for novel curcumin analogues, we synthesized and characterized 3,4-bis(2-hydroxybenzylidene)-4-piperidone [13]. In this work, we prepared and characterized N-benzyl-(3E,5E)-3,5-bis(2-hydroxybenzylidene)-4-piperidone.
Experimental Section
Synthesis of N-Benzyl-(3E,5E)-3,5-bis(2-hydroxybenzylidene)-4-piperidone (3)
Melting points were determined on an Electrothermal melting point apparatus and are uncorrected. The UV spectra were obtained on a UV Ultraspec 3000 Pro spectrophotometer. The IR spectra were recorded on a Perkin-Elmer 1760X FT-IR (Waltham, MA, USA) in KBr. The mass spectra were recorded with a JEOL JMS-700 (Tokyo, Japan) and a SynaptG2 mass spectrometer (Waters, Milford, MA, USA). 1H and 13C-NMR spectra were recorded with an Agilent DD2 system (Santa Clara, CA, USA) operating at 500 (1H) and 125 (13C) MHz, using residual (δH 7.26) and deuterated solvent (δC 77.0) peaks of CDCl3 as reference standards. For synthesis used Mas-II Sineo Microwave. Chromatographic separations were carried out on silica gel 60 (Merck, Darmstadt, Germany). TLC plates were precoated with silica GF254 (Merck, 0.25 mm) and detection was achieved by spraying with 10% H2SO4 in ethanol, followed by heating.
The title compound was synthesized by mixing the corresponding N-benzyl-4-piperidone (1.89 g, 0.01 mol), 2-hydroxybenzaldehyde (2.44 g, 0.02 mol), 40% aq. NaOH (0.7 mL) and 95% EtOH (5 mL) and was stirred at room temperature for 30 minutes, according to the partially modified procedure of a previous report [9], as shown in Scheme 1. The reaction mixture was subjected to microwave irradiation for 3 min at a power of 180 W and temperature of 60 °C. The reaction product was cooled and cold water was added. The precipitate formed was filtered and recrystallized from mixture of ethyl acetate–methanol to afford 3 (3.58 g, 90%) as an orange crystal, m.p: 143–144 °C. UV (MeOH) λmax: 306 nm (ε 4,600) and 357 nm (ε 5,200).
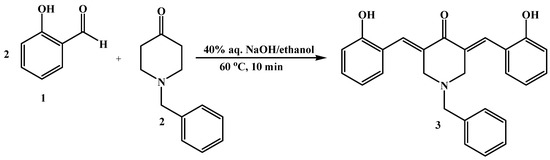
Scheme 1.
The synthesis of N-benzyl-(3E,5E)-3,5-bis(2-hydroxybenzylidene)-4-piperidone.
IR (KBr) νmax cm−1: 3235, 3064, 1706, 1661 and 1604.
1H-NMR (Agilent DD2, 500 MHz, CDCl3): δ (ppm) 8.90 (2H, s), 8.00 (2H, s), 7.40 (2H, d, J = 8.0 Hz), 6.90 (2H, d, J = 7.5 Hz), 6.80 (2H, d, J = 8.0 Hz), 6.70 (2H, d, J = 7.5 Hz), 3.83 (2H, s), 3.71 (4H, s).
13C-NMR (125 MHz, CDCl3): δ (ppm) 187.4, 157.6, 139.1, 134.0, 132.0, 131.3, 131.0, 129.8, 129.1, 127.9, 123.4, 120.1, 116.6, 62.1, 55.6.
HR-ESI-TOFMS: calculated for C26H24NO3 m/z 398.1756, found [M + H]+, m/z 398.1750.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
This research was partially supported by Directorate General of Higher Education, Ministry of Educatioan and Culture, Indonesia (2012-2013, by YE).
Author Contributions
Unang Supratman and Adel Zamri designed the whole experiment and contributed to the manuscript. Yoshihito Shiono measured the NMR and HR-ESI-TOFMS spectra. Yum Eryanti and Tati Herlina synthesize a new curcumin analog and wrote the manuscript. Khalijah Awang and Siti Nadiah Abdul Halim analyzed the NMR and HR-ESI-TOFMS spectra. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interst.
References
- Dandia, A.; Jain, A.K.; Sharma, S. An efficient and highly selective approach for the construction of novel dispiro heterocycles in guanidine-based task-specific [TMG][Ac] ionic liquid. Tetrahedron Lett. 2012, 53, 5859–5863. [Google Scholar] [CrossRef]
- Rostom, S.A.F.; Hassan, G.S.; El-Subbagh, H.I. Synthesis and biological evaluation of some polymethoxylated fused pyridine ring system as antitumor agents. Arch. Pharm. 2009, 342, 484–590. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Nakamura, T.; Iyoki, S.; Fujiwara, A.; Watanabe, Y.; Mohri, K.; Isobe, K.; Ono, K.; Yano, S. Elucidation of anti-allergic activities of curcumin-related compounds with a special reference to their anti-oxidative activities. J. Pharm. Bull. 2005, 28, 1438–1443. [Google Scholar] [CrossRef]
- Ranjith, K.R.; Subbu, P.; Palaniappan, S.; Perumal, Y.; Dharmarajan, S. An atom efficient, solvent-free, green synthesis and antimycobacterial evaluation of 2-amino-6-methyl-4-aryl-8-[(E)-arylmethylidene]-5,6,7,8-tetrahydro-4H-pyrano[3,2-c]pyridine-3-carbonitriles. Bioorg. Med. Chem. Lett. 2007, 17, 6459–6462. [Google Scholar]
- Babasaheb, Y.; Sebastian, T.; Rhonda, J.R.; Schumacher, N.; Diederich, Somer-Edgar, Tiffany, J.; Lesley, L. Synthesis and cytotoxic potential of heterocyclic cyclohexanone analogues of curcumin. Bioorg. Med. Chem. 2010, 18, 6701–6707. [Google Scholar]
- Reddy, B.V.; Sundari, J.S.; Balamurugan, E.; Menon, V.P. Prevention of nicotine and streptozotocin treatment induced circulatory oxidative stress by bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione in diabetic rats. Mol. Cell. Biochem. 2009, 331, 127–331. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Insuasty, B.; Becerra, D.; Quiroga, J.; Abonia, R.; Nogueras, M.; Cobo, J. Microwave-assisted synthesis of pyrimido[4,5-b][1,6]naphthyridin-4(3H)-ones with potential antitumor activity. Eur. J. Med. Chem. 2013, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Cai, Y.; Wang, J.; Weng, B.; Tang, Q.; Chen, X.; Pan, Z.; Liang, G.; Yang, S. Discovery and evaluation of piperid-4-one-containing mono-carbonyl analogs of curcumin as anti-inflammatory agents. Bioorg. Med. Chem. 2013, 21, 3959–3065. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Cai, Y.; He, X.; Li, J.; Zhang, L.; Wu, J.; Zhao, Y.; Yang, S.; Li, X.; Li, W.; Liang, G. Synthesis and anti-inflammatory evaluation of novel mono-carbonyl analogs of curcumin in LPS-stimulated RAW 264.7 macrophages. Eur. J. Med. Chem. 2010, 45, 5773–5780. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.M.; Cui, X.; Wu, R.; Dong, W.; Zhou, M.; Hu, M.; Simms, H.H.; Wang, P. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-γ. Crit. Care. Med. 2006, 34, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.; Dandavati, A.; Lee, M.; Tzou, S.; Savagian, M.; Brien, K. M.; Satam, V.; Patil, P.; Lee, M. Synthesis, cytotoxicity, and structure-activity insight of NH- and N-methyl-3,5-bis(arylidenyl)-4-piperidones. Med. Chem. Res. 2013, 22, 5588–5597. [Google Scholar] [CrossRef]
- Eryanti, Y.; Herlina, T.; Zamri, A.; Halim, S.A.N.; Shiono, Y.; Syah, Y.M.; Awang, K.; Supratman, U. 3,5-Bis(2-hydroxybenzylidene)piperidin-4-one. Molbank 2014, 2014, M825. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).