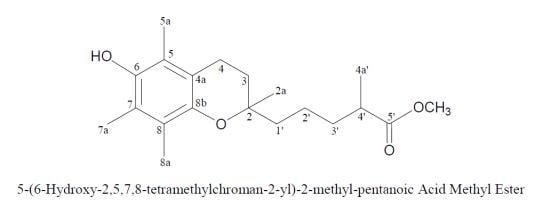
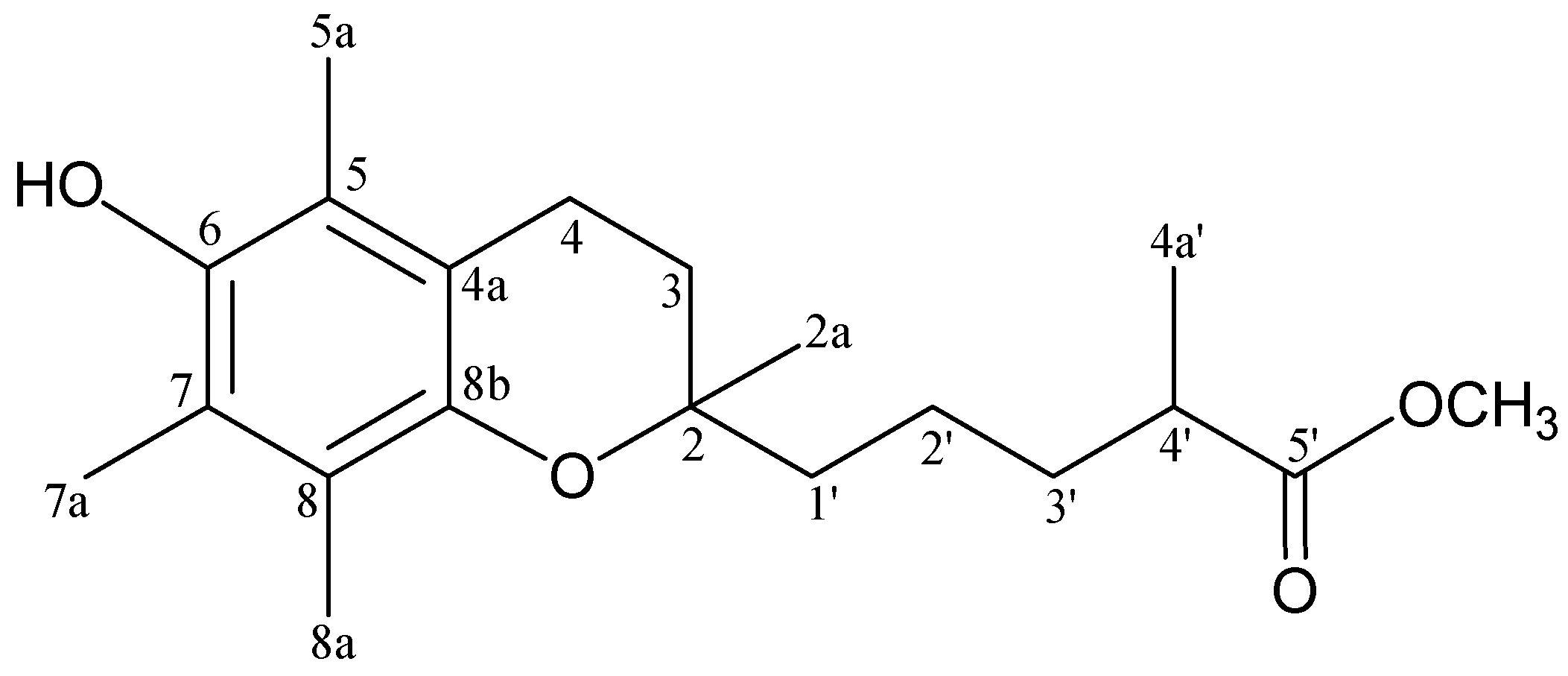
5-(6-Hydroxy-2,5,7,8-tetramethylchroman-2-yl)-2-methyl-pentanoic Acid Methyl Ester
Abstract
:Introduction
Results and Discussion
Experimental
General
Animal Material
Extraction and Isolation
Generation of Superoxide Anions by Human Neutrophils
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
Author Contributions
Conflict of Interest
References
- Verseveldt, J. Octocorallia from North-Western Madagascar (Part II). Zool. Verh. 1971, 117, 1–73. [Google Scholar]
- Chen, K.-H.; Dai, C.-F.; Lu, M.-C.; Li, J.-J.; Chen, J.-J.; Chang, Y.-C.; Su, Y.-D.; Wang, W.-H.; Sung, P.-J. Secondary Metabolites from the Soft Coral Sinularia arborea. Mar. Drugs 2013, 11, 3372–3380. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-H.; Dai, C.-F.; Hwang, T.-L.; Chen, C.-Y.; Li, J.-J.; Chen, J.-J.; Wu, Y.-C.; Sheu, J.-H.; Wang, W.-H.; Sung, P.-J. Discovery of novel diterpenoids from Sinularia arborea. Mar. Drugs 2014, 12, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Chen, K.-H.; Dai, C.-F.; Hwang, T.-L.; Wang, W.-H.; Wen, Z.-H.; Wu, Y.-C.; Sung, P.-J. New cembranoids from the soft coral Sinularia arborea. Nat. Prod. Commun. 2014, 9, 361–362. [Google Scholar] [PubMed]
- Pope, S.A.S.; Burtin, G.E.; Clayton, P.T.; Madge, D.J.; Muller, D.P.R. New synthesis of (±)-α-CMBH and its confirmation as a metabolite of α-tocopherol (vitamin E). Bioorg. Med. Chem. 2001, 9, 1337–1343. [Google Scholar] [CrossRef]
- Yang, S.-C.; Chung, P.-J.; Ho, C.-M.; Kuo, C.-Y.; Hung, M.-F.; Huang, Y.-T.; Chang, W.-Y.; Chang, Y.-W.; Chan, K.-H.; Hwang, T.-L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-P.; Hsieh, P.-W.; Chang, Y.-J.; Chung, P.-J.; Kuo, L.-M.; Hwang, T.-L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
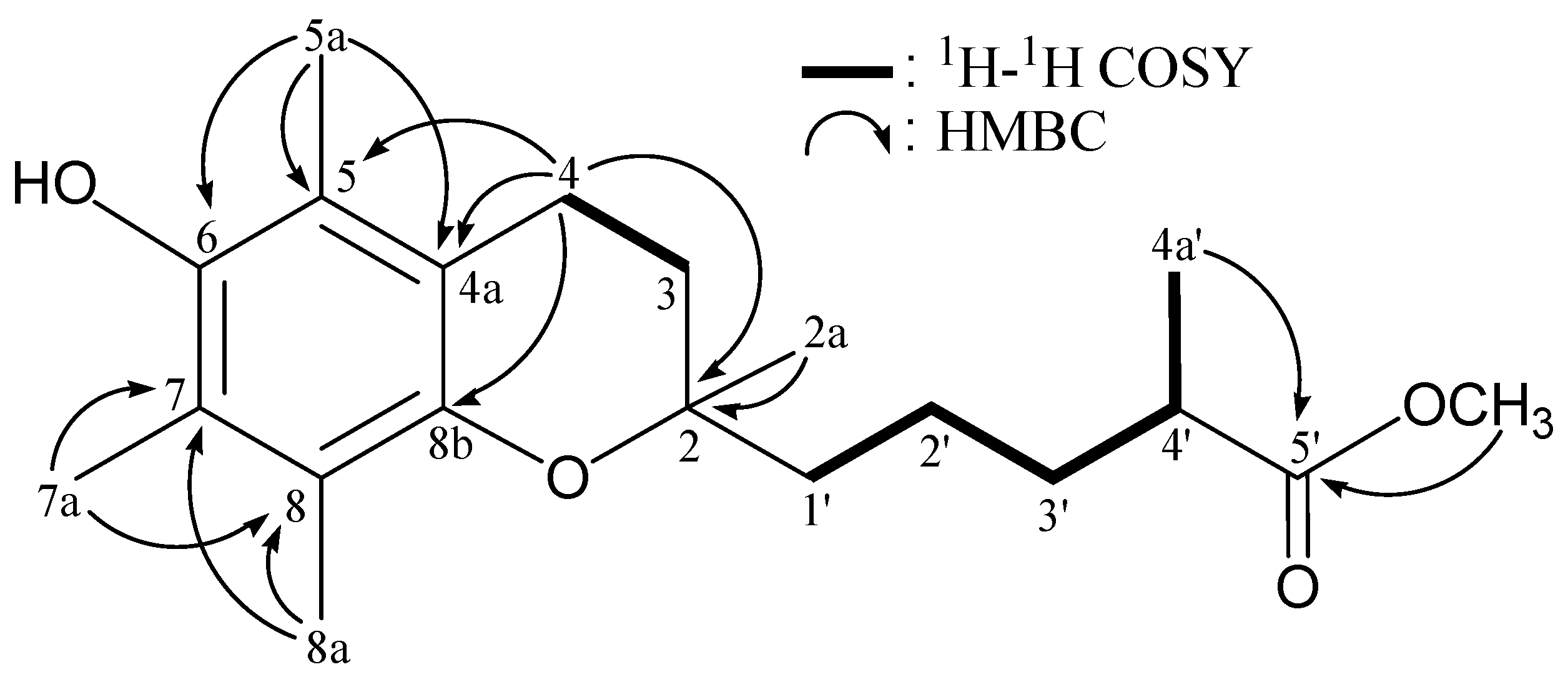
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC (H→C) | ||
|---|---|---|---|---|---|---|
| 2 | 74.25 | C | ||||
| 2a | 1.22 s | 23.67 | 23.65 | CH3 | C-2, -3, -1', | |
| 3 | 1.78 m | 31.55 | CH2 | H2-4 | C-4 | |
| 4 | 2.60 t (6.8) | 20.69 | CH2 | H2-3 | C-2, -3, -4a, -5, -8b | |
| 4a | 118.48 | C | ||||
| 5 | 117.25 | C | ||||
| 5a | 2.11 s | 11.26 | CH3 | C-4a, -5, -6 | ||
| 6 | 144.59 | C | ||||
| 7 | 121.04 | C | ||||
| 7a | 2.10 s | 11.75 | CH3 | C-7, -8 | ||
| 8 | 122.58 | C | ||||
| 8a | 2.16 s | 12.19 | CH3 | C-7, -8 | ||
| 8b | 145.40 | C | ||||
| 1' | 1.59 m, 1.51 m | 39.35 | 39.31 | CH2 | H2-2' | n.o. a |
| 2' | 1.44 m | 21.30 | 21.26 | CH2 | H2-1', H2-3' | n.o. |
| 3' | 1.66 m, 1.42 m | 34.20 | CH2 | H2-2', H-4' | n.o. | |
| 4' | 2.46 sext (5.6) | 39.40 | 39.36 | CH | H2-3', H3-4a' | n.o. |
| 4a' | 1.15 d (6.8) | 17.06 | 17.02 | CH3 | H-4' | C-3', -4', -5' |
| 5' | 177.28 | 177.24 | C | |||
| 5'-OCH3 | 3.67 s | 51.45 | 51.44 | CH3 | C-5' | |
| 6-OH | 4.20 br s | |||||
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, K.-H.; Dai, C.-F.; Hwang, T.-L.; Sung, P.-J. 5-(6-Hydroxy-2,5,7,8-tetramethylchroman-2-yl)-2-methyl-pentanoic Acid Methyl Ester. Molbank 2014, 2014, M822. https://doi.org/10.3390/M822
Chen K-H, Dai C-F, Hwang T-L, Sung P-J. 5-(6-Hydroxy-2,5,7,8-tetramethylchroman-2-yl)-2-methyl-pentanoic Acid Methyl Ester. Molbank. 2014; 2014(2):M822. https://doi.org/10.3390/M822
Chicago/Turabian StyleChen, Kuan-Hua, Chang-Feng Dai, Tsong-Long Hwang, and Ping-Jyun Sung. 2014. "5-(6-Hydroxy-2,5,7,8-tetramethylchroman-2-yl)-2-methyl-pentanoic Acid Methyl Ester" Molbank 2014, no. 2: M822. https://doi.org/10.3390/M822
APA StyleChen, K.-H., Dai, C.-F., Hwang, T.-L., & Sung, P.-J. (2014). 5-(6-Hydroxy-2,5,7,8-tetramethylchroman-2-yl)-2-methyl-pentanoic Acid Methyl Ester. Molbank, 2014(2), M822. https://doi.org/10.3390/M822