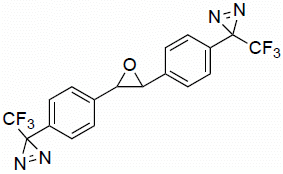
2,3-Bis(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenyl)oxirane
Abstract
:Experimental
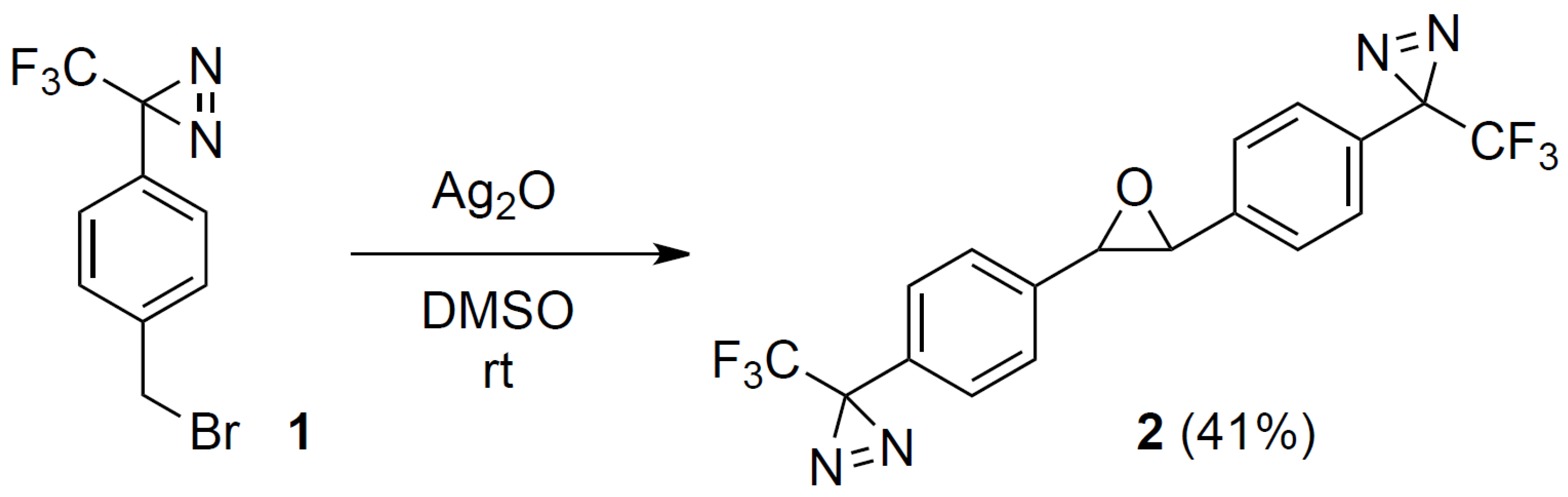
Synthesis of 2,3-bis(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenyl)oxirane (2)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
Conflicts of Interest
References and Notes
- Hashimoto, M.; Hatanaka, Y. Recent progress in diazirine-based photoaffinity labeling. Eur. J. Org. Chem. 2008, 2008, 2513–2523. [Google Scholar] [CrossRef]
- Blencowe, A.; Blencowe, C.; Cosstick, K.; Hayes, W. A carbene insertion approach to functionalized poly(ethylene oxide)-based gels. React. Funct. Polym. 2008, 68, 868–875. [Google Scholar] [CrossRef]
- Murai, Y.; Wang, L.; Muto, Y.; Sakihama, Y.; Hashidoko, Y.; Hatanaka, Y.; Hashimoto, M. Simple and stereocontrolled preparation of benzoylated phenylalanine using Friedel–Crafts reaction in trifluoromethanesulfonic acid for photoaffinity labeling. Heterocycles 2013, 87, 2119–2126. [Google Scholar] [CrossRef]
- Wong, F.M.; Chan, Y.M.; Chen, D.X.; Wu, W. Convenient one-pot synthesis of trans-1,2-diaryloxiranes from the direct coupling of benzyl halides. Tetrahedron Lett. 2010, 51, 6649–6650. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Lo, M.M.-C.; Fu, G.C. Planar-chiral pyridine N-Oxides, a new family of asymmetric catalysts: Exploiting an η5-C5Ar5 ligand to achieve high enantioselectivity. J. Am. Chem. Soc. 2001, 123, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Ando, F.; Koketsu, J. Generation of sulfur ylides from sulfonium salts and their reactions. Comparative study of electrochemical reduction with the base method and mechanism elucidation by the MO method. Bull. Chem. Soc. Jpn. 2003, 76, 2155–2165. [Google Scholar] [CrossRef]
- Brunner, J.; Senn, H.; Richards, F.M. 3-Trifluoromethyl-3-phenyldiazirine. A new carbene generating group for photolabeling reagents. J. Biol. Chem. 1980, 255, 3313–3318. [Google Scholar] [PubMed]
- Nakashima, H.; Hashimoto, M.; Sadakane, Y.; Tomohiro, T.; Hatanaka, Y. Simple and Versatile method for tagging phenyldiazirine photophores. J. Am. Chem. Soc. 2006, 128, 15092–15093. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, L.; Yoshida, T.; Okamoto, M.; Sakihama, Y.; Hashidoko, Y.; Hashimoto, M. 2,3-Bis(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenyl)oxirane. Molbank 2014, 2014, M816. https://doi.org/10.3390/M816
Wang L, Yoshida T, Okamoto M, Sakihama Y, Hashidoko Y, Hashimoto M. 2,3-Bis(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenyl)oxirane. Molbank. 2014; 2014(1):M816. https://doi.org/10.3390/M816
Chicago/Turabian StyleWang, Lei, Takuma Yoshida, Masashi Okamoto, Yasuko Sakihama, Yasuyuki Hashidoko, and Makoto Hashimoto. 2014. "2,3-Bis(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenyl)oxirane" Molbank 2014, no. 1: M816. https://doi.org/10.3390/M816
APA StyleWang, L., Yoshida, T., Okamoto, M., Sakihama, Y., Hashidoko, Y., & Hashimoto, M. (2014). 2,3-Bis(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenyl)oxirane. Molbank, 2014(1), M816. https://doi.org/10.3390/M816