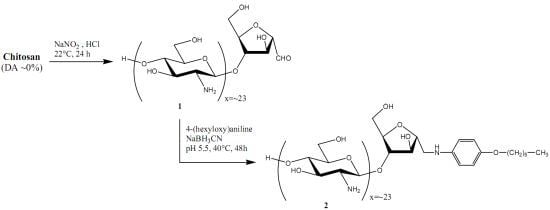
4-(Hexyloxy)aniline-linked chitooligosaccharide-2,5-anhydro-D-mannofuranose
Abstract
:Results and Discussion
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Conflicts of Interest
References
- Domard, A.; Domard, M. Chitosan: Structure-Properties Relationship and Biomedical Applications. In Polymeric Biomaterials; Dumitriu, S., Ed.; Marcel Dekker: New York, USA, 1994; pp. 187–212. [Google Scholar]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Harris, R.; Heras, A. Chitosan amphiphilic derivatives: Chemistry and applications. Curr. Org. Chem. 2010, 14, 308–330. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan: A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Kim, S.-K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Trombotto, S.; Ladavière, C.; Delolme, F.; Domard, A. Chemical preparation and structural characterization of a homogeneous series of chitin/chitosan oligomers. Biomacromolecules 2008, 9, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, characterization and applications. Polym. Sci. Ser. A Polym. Phys. 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sørlie, M.; Vårum, K.M.; Eijsink, V.G.H. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocolloids 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Rasmussen, M.O.; Hogg, B.; Bono, J.-J.; Samain, E.; Driguez, H. New access to lipo-chitooligosaccharides nodulations factors. Org. Biomol. Chem. 2004, 2, 1908–1910. [Google Scholar] [CrossRef] [PubMed]
- Alba, M.; Marmuse, L.; Delolme, F.; Vors, J.P.; Ladavière, C.; Trombotto, S. Access to tetra-N-acetyl-chitopentaose by chemical N-acetylation of glucosamine pentamer. Carbohydr. Polym. 2013, 98, 770–777. [Google Scholar]
- Guerry, A.; Bernard, J.; Samain, E.; Fleury, E.; Cottaz, S.; Halila, S. Aniline-catalyzed reductive amination as a powerful method for the preparation of reducing end-"Clickable" chitooligosaccharides. Bioconjugate Chem. 2013, 24, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Tommeraas, K.; Varum, K.M.; Christensen, B.E.; Smidsrod, O. Preparation and characterization of oligosaccharides produced by nitrous acid depolymerization of chitosans. Carbohydr. Res. 2001, 333, 137–144. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Salim, E.; Galais, A.; Trombotto, S. 4-(Hexyloxy)aniline-linked chitooligosaccharide-2,5-anhydro-D-mannofuranose. Molbank 2014, 2014, M815. https://doi.org/10.3390/M815
Salim E, Galais A, Trombotto S. 4-(Hexyloxy)aniline-linked chitooligosaccharide-2,5-anhydro-D-mannofuranose. Molbank. 2014; 2014(1):M815. https://doi.org/10.3390/M815
Chicago/Turabian StyleSalim, Emil, Alice Galais, and Stéphane Trombotto. 2014. "4-(Hexyloxy)aniline-linked chitooligosaccharide-2,5-anhydro-D-mannofuranose" Molbank 2014, no. 1: M815. https://doi.org/10.3390/M815
APA StyleSalim, E., Galais, A., & Trombotto, S. (2014). 4-(Hexyloxy)aniline-linked chitooligosaccharide-2,5-anhydro-D-mannofuranose. Molbank, 2014(1), M815. https://doi.org/10.3390/M815