Ethyl 7-Methyl-5-(4-methylphenyl)-3-oxo-2-{[3-(3,4-dichlorophenyl)-1-phenyl-1H-pyrazol-4-yl]methylidene}-2,3-dihydro-5H-[1,3]thiazolo[3,2-a]pyrimidine-6-carboxylate
Abstract
:Introduction
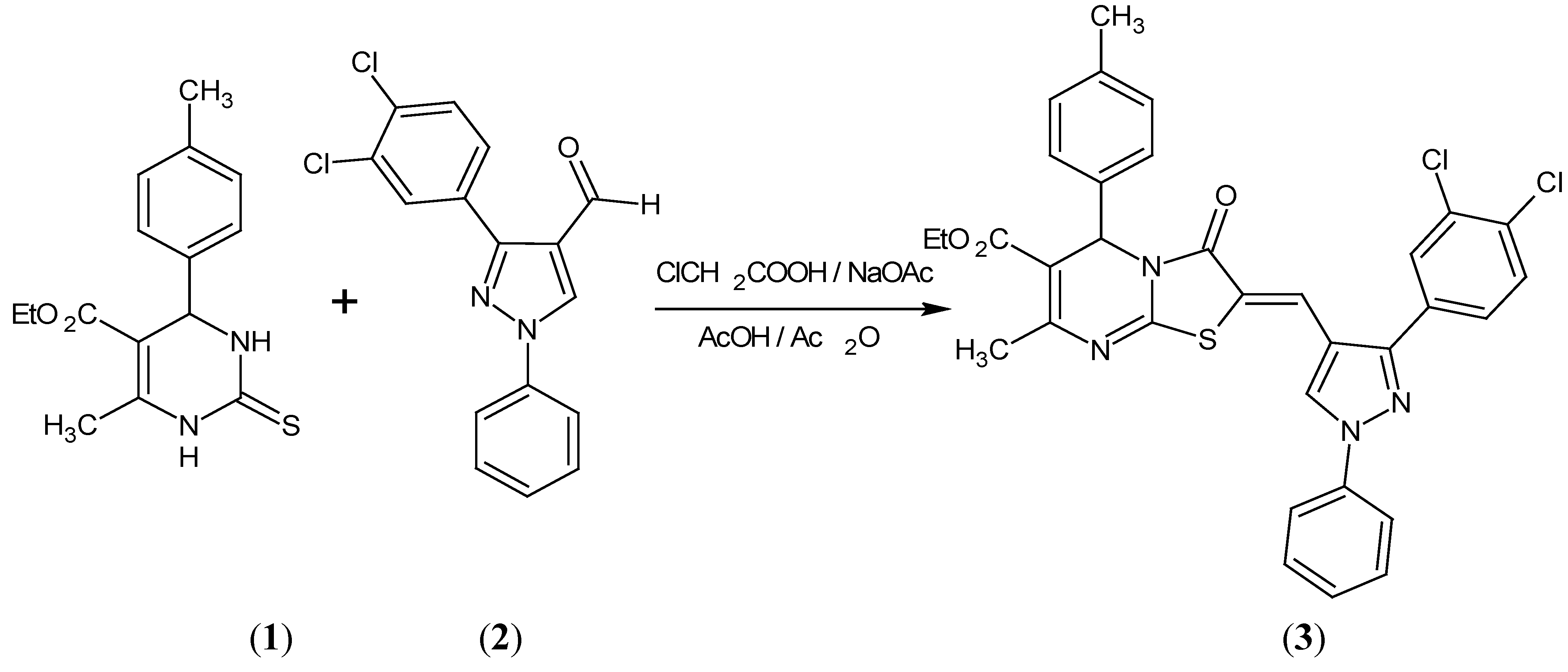
Results and Discussion
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
References
- Kappe, C.O. 100 Years of the biginelli dihydropyrimidine synthesis. Tetrahedron 1993, 49, 6937–6963. [Google Scholar] [CrossRef]
- Atwal, K.S.; Swanson, B.N.; Unger, S.E.; Floyd, D.M.; Moreland, S.; Hedberg, A.; O’Reilly, B.C. Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. J. Med. Chem. 1991, 34, 806–811. [Google Scholar] [PubMed]
- Wilson, K.J.; Illig, C.R.; Subhasinghe, N.; Hoffmann, J.B.; Rudolph, M.J.; Soll, R.; Molloy, C.J.; Bone, R.; Green, D.; Randall, T.; et al. Synthesis of thiophene-2-carboxamidines containing 2-amino-thiazoles and their biological evaluation as urokinase inhibitors. Bioorg. Med. Chem. Lett. 2001, 11, 915–918. [Google Scholar] [CrossRef]
- Berlin, K.D.; Herd, M.D. Novel 2-amino-4-aryl-substituted and 2-amino-4,5-disubstituted-Thiazoles. Proc. Okla. Acad. Sci. 1991, 71, 29–33. [Google Scholar]
- Holla, B.S.; Malini, K.V.; Rao, B.S.; Sarojini, B.K.; Kumari, N.S. Synthesis of some new 2,4-disubstituted thiazoles as possible antibacterial and anti-inflammatory agents. Eur. J. Med. Chem. 2003, 38, 313–318. [Google Scholar] [CrossRef]
- Zhi, H.; Chen, L.; Zhang, L.; Liu, S.; Cheong Wan, D.C.; Lin, H.; Hu, C. Design, synthesis, and biological evaluation of 5H-thiazolo[3,2-a]pyrimidine derivatives as a new type of acetylcholinesterase inhibitors. ARKIVOC 2008, xiii, 266–277. [Google Scholar]
- Zhi, H.; Chen, L.; Zhang, L.; Liu, S.; Wan, D.C.C.; Lin, H.; Hu, C. Design, synthesis, and biological evaluation of 5H-thiazolo[3,2-a]pyrimidine-6-carboxylic acid ethyl ester derivatives as a novel series of acetylcholinesterase inhibitors. Chem. Res. Chin. Univ. 2009, 25, 332–337. [Google Scholar]
- Aly, M.F.; El-Naggar, G.M.; El-Emary, T.I.; Grigg, R.; Metwally, S.A.M.; Sivagnanam, S. X=Y–ZH Compounds as potential 1,3-Dipoles: Part 41. Azomethine ylide formation from the reactions of α-amino acids and esters with alloxan (strecker degradation) and with 1-phenyl-3-methylpyrazolin-4,5-dione. Tetrahedron. 1994, 50, 895–906. [Google Scholar] [CrossRef]
- Jung, M.E.; Min, S.J.; Houk, K.N.; Ess, D. Synthesis and relative stability of 3,5-diacyl-4,5-dihydro-1H-pyrazoles prepared by dipolar cycloaddition of enones and α-diazoketones. J. Org. Chem. 2004, 69, 9085–9089. [Google Scholar] [CrossRef] [PubMed]
- El-Emary, T.I. Synthesis of newly substituted pyrazoles and substituted pyrazolo [3,4-b]pyridines based on 5-amino-3-methyl-1-phenylpyrazole. J. Chin. Chem. Soc. 2007, 54, 507–518. [Google Scholar] [CrossRef]
- Ali, M.I.; El-fotooh, A.; Hamman, G.; Mohamad, S.F. Synthesis and reactions of 2,3-dihydro-5-aryl-5h,6h-thiazolo[3,2-b]-2,4-diazafluorene-3,6-diones of potential biological activities. Phosphorus Sulfur Silicon Relat. Elem. 1988, 39, 211–216. [Google Scholar] [CrossRef]
- Abdel-Gawad, S.M.; El-Gaby, M.S.A.; Ghorab, M.M. Synthesis and antifungal activity of novel pyrano[2',3':4,5]thiazolo[2,3-b]quinazolines, pyrido[2',3':4,5]thiazolo[2,3-b]quinazolines and pyrazolo[2',3':4,5]thiazolo[2,3-b]quinazolines. Il Farmaco 2000, 55, 287–292. [Google Scholar] [CrossRef]
- Mobinikhaledi, A.; Foroughifar, N.; Ebrahimi, S.; Rahimi, F.; Zandi, F. Synthesis of some novel 2-arylidene thiazoloquinazolinone derivatives via one-pot, three-component reaction. Phosphorus, Sulfur Silicon Relat. Elem. 2011, 186, 457–463. [Google Scholar] [CrossRef]
- Russowsky, D.; Lopes, F.A.; da Silva, V.S.S.; Canto, K.F.S.; D’Oca, M.G.M.; Godoi, M.N. Multicomponent Biginelli’s synthesis of 3,4-dihydropyrimidin-2(1H)-ones promoted by SnCl2.2H2O. J. Braz. Chem. Soc. 2004, 15, 165–168. [Google Scholar]
- Kira, M.A.; Abdel-Rahman, M.O.; Gadalla, K.Z. The Vilsmeier-haack reaction–III cyclization of hydrazones to pyrazoles. Tetrahedron Lett. 1969, 10, 109–110. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Viveka, S.; Dinesha; Laxmeshwar, S.S.; Nagaraja, G.K. Ethyl 7-Methyl-5-(4-methylphenyl)-3-oxo-2-{[3-(3,4-dichlorophenyl)-1-phenyl-1H-pyrazol-4-yl]methylidene}-2,3-dihydro-5H-[1,3]thiazolo[3,2-a]pyrimidine-6-carboxylate. Molbank 2012, 2012, M776. https://doi.org/10.3390/M776
Viveka S, Dinesha, Laxmeshwar SS, Nagaraja GK. Ethyl 7-Methyl-5-(4-methylphenyl)-3-oxo-2-{[3-(3,4-dichlorophenyl)-1-phenyl-1H-pyrazol-4-yl]methylidene}-2,3-dihydro-5H-[1,3]thiazolo[3,2-a]pyrimidine-6-carboxylate. Molbank. 2012; 2012(3):M776. https://doi.org/10.3390/M776
Chicago/Turabian StyleViveka, Shivapura, Dinesha, Sandeep Sadananda Laxmeshwar, and Gundibasappa Karikannar Nagaraja. 2012. "Ethyl 7-Methyl-5-(4-methylphenyl)-3-oxo-2-{[3-(3,4-dichlorophenyl)-1-phenyl-1H-pyrazol-4-yl]methylidene}-2,3-dihydro-5H-[1,3]thiazolo[3,2-a]pyrimidine-6-carboxylate" Molbank 2012, no. 3: M776. https://doi.org/10.3390/M776
APA StyleViveka, S., Dinesha, Laxmeshwar, S. S., & Nagaraja, G. K. (2012). Ethyl 7-Methyl-5-(4-methylphenyl)-3-oxo-2-{[3-(3,4-dichlorophenyl)-1-phenyl-1H-pyrazol-4-yl]methylidene}-2,3-dihydro-5H-[1,3]thiazolo[3,2-a]pyrimidine-6-carboxylate. Molbank, 2012(3), M776. https://doi.org/10.3390/M776




