Abstract
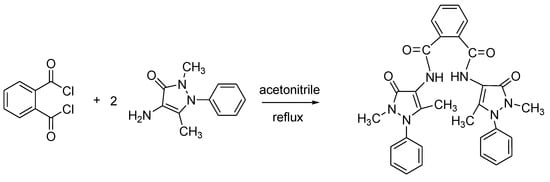
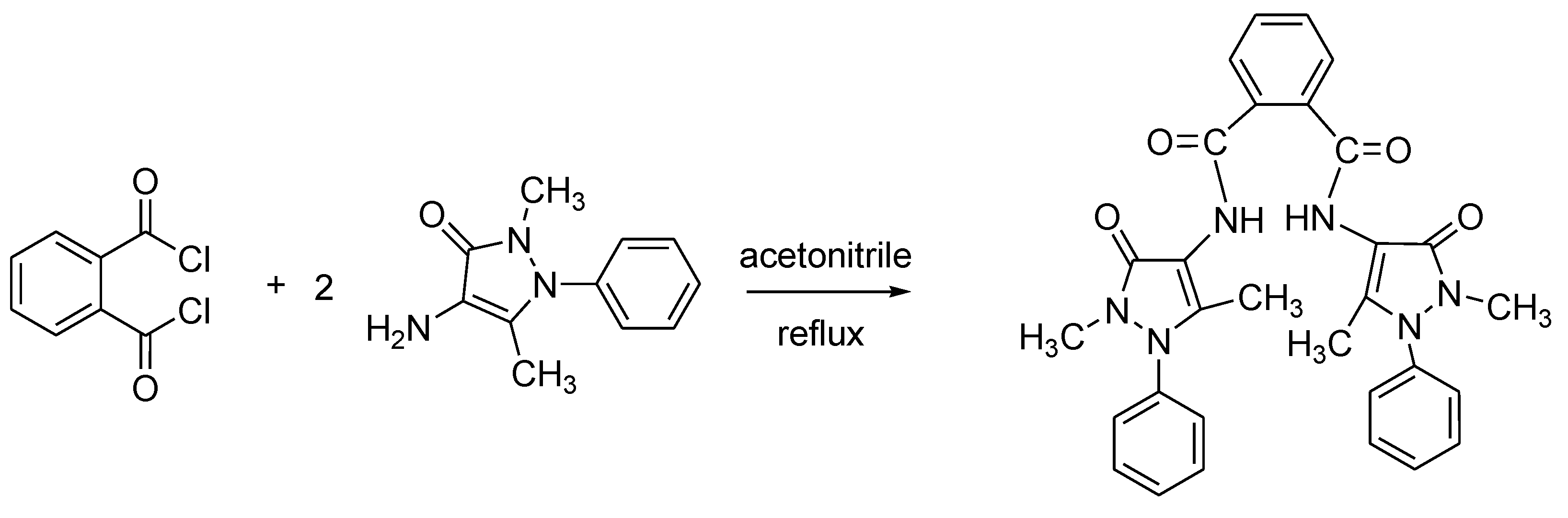
The title compound, N,N’-bis-(2,5-dimethyl-3-oxo-1-phenyl-2,3-dihydro-1H-pyrazol-4-yl)phthalamide has been synthesized by reactions of o-phthaloyl chloride with 4-amino-2,5-dimethyl-1-phenyl-3-oxo-1H-pyrazolone in acetonitrile. The structure of the new compound was characterized by FT-IR, 1H NMR, 13C NMR and mass spectroscopic techniques. Physical parameters of the compound such as melting point, solubility, λmax were examined.
Introduction
The pyrazole ring has been known as an important framework in a large number of compounds possessing pharmaceutical and agrochemical properties [1,2,3]. In addition, some substituted pyrazolines have constituted an important class of conjugated nitrogen-containing fluorescent compounds [4]. Functionalized N-arylpyrazoles have been shown to exhibit antihyperglycemic, analgesic, anti-inflammatory, sedative, and hypnotic activities [5]. Some phthalic acid diamide derivatives have been used as agricultural and horticultural insecticides [6].
In view of this, we report the convenient preparation of a new representative of this type of compounds, combining both of the above-mentioned structural elements.
Experimental
Reagents and Techniques
The 1H and 13C NMR spectra were recorded on a Bruker AVANCE DPX NMR spectrometer operating at 400 and 101.6 MHz. Infrared absorption spectra were obtained from a Perkin Elmer BX-II Spectrum 100 and are reported in cm−1 units. Melting points were measured on an Electro Thermal IA 9100 apparatus using a capillary tube. LC mass spectra were obtained on an AGILENT 1100 MSD spectrometer with an ion source temperature of 240 °C. o-Phthaloyl chloride, 4-amino-2,5-dimethyl-1-phenyl-3-oxo-1H-pyrazolone, acetonitrile, tetrahydrofuran and acetone were purchased from Merck.
Preparation of the Title Compound
A solution of o-phthaloyl chloride (1.01 g, 5 mmol) in acetonitrile (20 mL) was added dropwise to a solution of 4-amino-2,5-dimethyl-1-phenyl-3-oxo-1H-pyrazolone (2.03 g, 10 mmol) in acetonitrile (20 mL). The mixture was refluxed with stirring for 3 h. The progress of the reaction was controlled by TLC. After cooling the reaction mixture to ambient temperature, the solid was filtered off and recrystallized from tetrahydrofuran/acetone to give the product. The physical and spectral data of the compound are as follows: colorless crystal, yield 2.68 g (88%), melting point: 204–206 °C.
UV-Vis, λmax: 281 nm (in Me2SO);
FT-IR : νmax (cm−1): 3496 (N-H stretching), 3029 (aromatic C-H stretching), 2986 (C-H aliphatic), 1675 (amide, C=O stretching), 1783, 1713 (C=O, pyrazolone), 1490 (N-CO);
1H NMR (400 MHz, DMSO-d6) δ ppm: 6.43 (s, 2H, N-H), 8.01 (t, J = 4.3 Hz, 2H, Cphtaloyl-H), 7.95 (d, J = 3.8 Hz, 2H, Cphtaloyl-H); ), 7.92 (t, J = 5.1 Hz, 4H, Cphen.-H), 7.54 (t, J = 3.5 Hz, 2H, Cphen.-H), 7.39 (d, J = 3.2, 4H, Cphen.-H), 3.43 (s, 6H, N-CH3), 2.08 (s, 6H, C-CH3);
13C-NMR (100 MHz, DMSO-d6) δ ppm: 168.29 (C=O), 161.49 (C=O), 135.33, 132.81, 130.59 (Cphtaloyl), 136.21, 124.83, 128.69, 126.12 (Cphenyl), 154.90, 100.50 (Cpyrazolone), 35.83 (N-CH3), 10.87 (C-CH3);
Anal. Calcd. for C30H28N6O4: C, 67.15%; H, 5.26%; N, 15.66%. Found: C, 67.06%; H, 5.31%; N, 15.67%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
This work was supported by the Çanakkale Onsekiz Mart University Research Fund (BAP project 2009-119).
References
- Ali, M.A.; Siddiqui, M.S.A.A. Synthesis, structural activity relationship and anti-tubercular activity of novel pyrazoline derivatives. Eur. J. Med. Chem. 2007, 42, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Azarifar, D.; Shaebanzadeh, M. Synthesis and characterization of new 3,5-dinaphthyl substituted 2-pyrazolines and study of their antimicrobial activities. Molecules 2002, 7, 885–895. [Google Scholar] [CrossRef]
- Abunada, N.M.; Hassaneen, H.M.; Kandile, N.G.; Miqdad, O.M. Synthesis and biological activity of some new pyrazoline and pyrolo[3,4-c]pyrazole-4,6-dione derivatives: Reaction of nitrilimines with some dipolarophiles. Molecules 2008, 13, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Abbas, A.; Akhtar, M.N. Synthesis, characterization and fluoresent property evaluation of 1,3,5-triaryl-2-pyrazolines. Molecules 2011, 16, 7789–7802. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Kumar, H.; Khan, S.A. Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg. Med. Chem. Lett. 2008, 18, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.L.; Li, Y.-F.; Zhu, H.-J.; Zhao, L.; Xi, B.-B.; Ni, J.-P. Synthesis, insecticidal activity and structure activity relation of trifluoromethyl containing phthalic acid diamide structure. J. Agr. Food Chem. 2010, 58, 10999–11006. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).