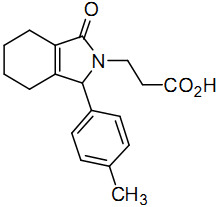
3-[1-(4-Methylphenyl)-3-oxo-1,3,4,5,6,7-hexahydro-2H-isoindol-2-yl]propanoic Acid
Abstract
: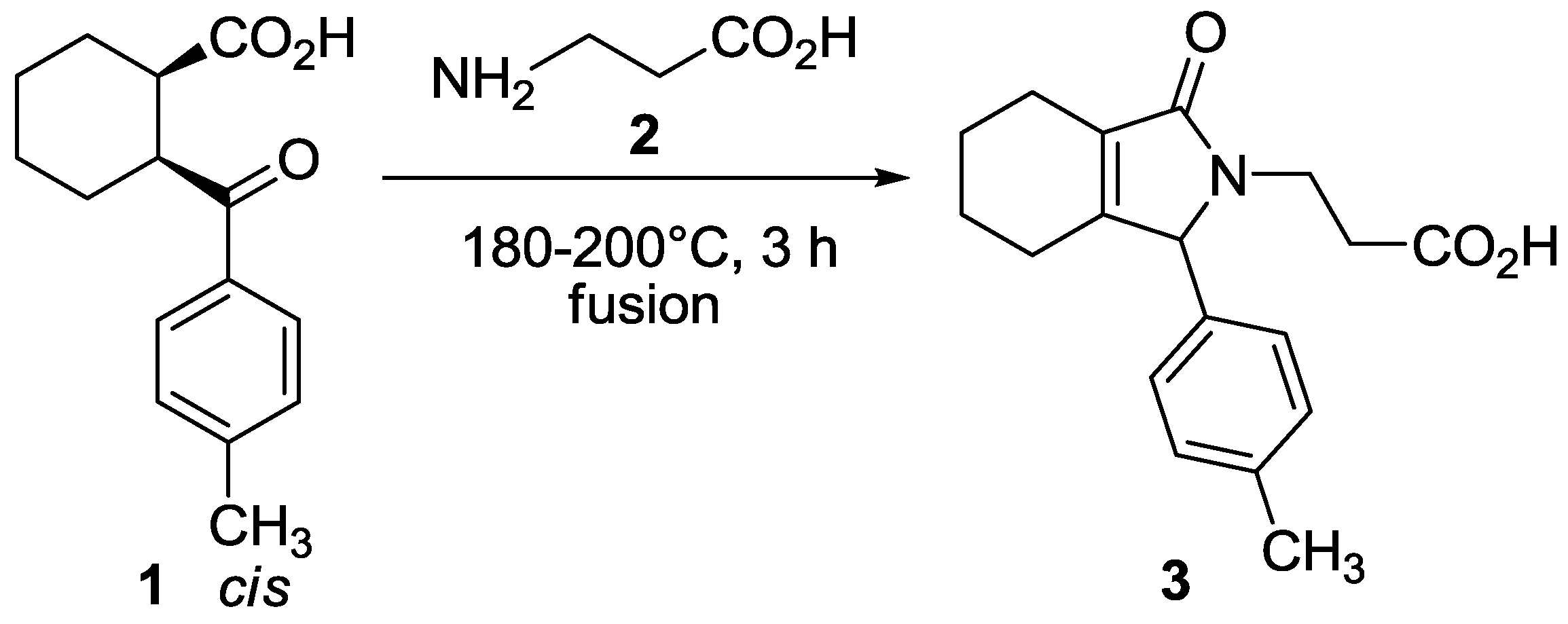
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References and Notes
- Nannini, G.; Giraldi, P.N.; Molgora, G.; Biasoli, G.; Spinelli, F.; Logemann, W.; Dradi, E.; Zanni, G.; Buttinoni, A.; Tommasini, R. New analgesic-anti-inflammatory drugs. 1-Oxo-2-substituted isoindoline derivatives. Arzneim. Forsch. 1973, 23, 1090–1100. [Google Scholar]
- Okazaki, K.; Oshima, E.; Obase, H.; Oiji, Y.; Nito, M.; Kubo, K. Isoindolin-1-one derivative and antiarrhythmic agent. Eur. Patent 0,273,401, 06 July 1988. [Chem. Abstr. 1989, 109, 170232p]. [Google Scholar]
- Csende, F.; Porkoláb, A.; Matíz, K.; Szabó, Z.; Csorvássy, I.; Frank, L. Preparation and antiarrhythmic activity of hexahydroisoindol-1-one derivatives. Sci. Pharm. 1999, 67, 149–158. [Google Scholar]
- Reyes, A.; Huerta, L.; Alfaro, M.; Navarrete, A. Synthesis and nootropic activity of some 2,3-dihydro-1H-isoindol-1-one derivatives structurally related with piracetam. Chem. Biodivers. 2010, 7, 2718–2726. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Fukuda, N. Effect of a new anxiolytic, DN-2327, on learning and memory in rats. Pharmacol. Biochem. Behav. 1992, 41, 573–579. [Google Scholar] [CrossRef]
- de Wit, H.; Vicini, L.; Haig, G.M.; Hunt, T.; Feltner, D. Evaluation of the abuse potential of pagoclone, a partial GABAA agonist. J. Clin. Psychopharmacol. 2006, 26, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Zsotér, T.T.; Hart, F.; Radde, I.C.; Endrenyi, L. Effect of chlorthalidone on blood vessels. J. Pharmacol. Exp. Ther. 1972, 180, 723–731. [Google Scholar] [PubMed]
- Zhuang, Z.-P.; Kung, M.P.; Mu, M.; Kung, H.F. Isoindol-1-one analogues of 4-(2'-methoxyphenyl)-1-[2'-[N-(2"-pyridyl)-p-iodobenzamido]ethyl]piperazine (p-MPPI) as 5-HT1A receptor ligands. J. Med. Chem. 1998, 41, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Hamprecht, D.; Micheli, F.; Tedesco, G.; Checchia, A.; Donati, D.; Petrone, M.; Terreni, S.; Wood, M. Isoindolone derivatives, a new class of 5-HT2C antagonists: Synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2007, 17, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Mukai, T.; Yamaguchi, E.; Goto, J.; Takagi, K. Smooth muscle relaxing drugs and guinea pig ileum. Jpn. J. Pharmacol. 1981, 37, 147–157. [Google Scholar] [CrossRef]
- Chiba, S.; Nishiyama, T.; Yamada, Y. The antinociceptive effects and pharmacological properties of JM-1232(-): A novel isoindoline derivative. Anesth. Analg. 2009, 108, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Kanamitsu, N.; Osaki, T.; Itsuji, Y.; Yoshimura, M.; Tsujimoto, H.; Soga, M. Novel water-soluble sedative-hypnotic agents: isoindolin-1-one derivatives. Chem. Pharm. Bull. 2007, 55, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Usifoh, C.O.; Lambert, D.M.; Wouters, J.; Scriba, G.K.E. Synthesis and anticonvulsant activity of N,N-phthaloyl derivatives of central nervous system inhibitory amino acids. Arch. Pharm. 2001, 334, 323–331. [Google Scholar] [CrossRef]
- Blaskó, G.; Gula, D.J.; Shamma, M. The phthalideisoquinoline alkaloids. J. Nat. Prod. 1982, 45, 105–122. [Google Scholar] [CrossRef]
- Valencia, E.; Freyer, A.J.; Shamma, M.; Fajardo, V. (±)-Nuevamine, an isoindoloisoquinoline alkaloid, and (±)-lennoxamine, an isoindolobenzazepine. Tetrahedron Lett. 1984, 25, 599–602. [Google Scholar] [CrossRef]
- Bentley, K.W. β-Phenylethylamines and the isoquinoline alkaloids. Nat. Prod. Rep. 1986, 3, 153–169. [Google Scholar] [CrossRef]
- Csende, F.; Szabó, Z.; Stájer, G. Synthesis and structural study of new saturated isoindol-1-one derivatives. Heterocycles 1993, 36, 1809–1821. [Google Scholar] [CrossRef]
- Stájer, G.; Csende, F.; Bernáth, G.; Sohár, P. Preparation and steric structure of tricyclic and tetracyclic saturated or partially saturated 1,3-heterocycles containing a saturated isoindolone moiety. Heterocycles 1994, 37, 883–890. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Csende, F.; Jekő, J.; Porkoláb, A. 3-[1-(4-Methylphenyl)-3-oxo-1,3,4,5,6,7-hexahydro-2H-isoindol-2-yl]propanoic Acid. Molbank 2011, 2011, M742. https://doi.org/10.3390/M742
Csende F, Jekő J, Porkoláb A. 3-[1-(4-Methylphenyl)-3-oxo-1,3,4,5,6,7-hexahydro-2H-isoindol-2-yl]propanoic Acid. Molbank. 2011; 2011(4):M742. https://doi.org/10.3390/M742
Chicago/Turabian StyleCsende, Ferenc, József Jekő, and Andrea Porkoláb. 2011. "3-[1-(4-Methylphenyl)-3-oxo-1,3,4,5,6,7-hexahydro-2H-isoindol-2-yl]propanoic Acid" Molbank 2011, no. 4: M742. https://doi.org/10.3390/M742
APA StyleCsende, F., Jekő, J., & Porkoláb, A. (2011). 3-[1-(4-Methylphenyl)-3-oxo-1,3,4,5,6,7-hexahydro-2H-isoindol-2-yl]propanoic Acid. Molbank, 2011(4), M742. https://doi.org/10.3390/M742