Abstract
We report herein the synthesis of 1,1,2,2-tetrakis[(benzoylamino)methyl]-hydrazine from (benzamidomethyl)triethylammonium chloride and hydrazine monohydrate in the presence of triethylamine in ethanol/aqueous media. The structure of the newly synthesized compound was characterized on the basis of 1H-NMR, 13C-NMR, IR and mass spectral data.
1. Introduction
The promising usefulness of the benzamidomethyl derivatives as biologically active products [1,2,3] and their implication for pro-drug design [4,5,6,7] have been previously reported. On the other hand, many hydrazine derivatives are known to exhibit significant biological activity and several compounds with hydrazine moiety were shown to be effective for treatment of tuberculosis, Parkinson’s disease, hypertension and widely used as antidepressant drugs [8,9]. The hydrazine moiety has been used for modification of peptides as well. For example, novel building blocks for peptide modification have been designed and synthesized to obtain β-sheet tripeptide mimics that comprise the azapeptide modification at one end [10]. Azapeptides, hydrazine-based peptidomimetics, were found to be potent agents against hepatitis [11], AIDS [12] and SARS [13]. In addition, hydrazines, particularly when they are tetrasubstituted, are an excellent source of radical cations [14,15,16,17,18]. Tetraalkylhydrazines are also employed in the study of single-electron transfer reactions [15,16,17,18], and as charge-transfer quenchers of singlet oxygen [14].
In the course of our continuous study on the benzamidomethylation reactions of N-containing compounds [19,20,21,22], we report herein the synthesis and spectral characterization of the novel 1,1,2,2-tetrakis[(benzoylamino)methyl]hydrazine.
2. Results and Discussion
The title compound was prepared by the reaction of a hydrazine monohydrate (2) in ethanol/aqueous (v/v = 2:1) solution with (benzamidomethyl)triethylammonium chloride (1) in the presence of a small quantity of triethylamine (pH > 9) at room temperature. In fact, hydrazine in the presence of a large excess of 1, leads to formation of two compounds in aqueous media in an overall yield of 93%. The ratio of the formed products, tetrakis[(benzoylamino)methyl]hydrazine (3) and 1,1,2-tris[(benzoylamino)methyl]hydrazine (4), was 1.2:1. In order to improve the yield of 3, the effect of the mole proportion of reactants and solvents was investigated. It was found that the corresponding tetrabenzamidomethylated product was obtained predominantly by increasing the amount of 1 and utilizing an ethanol-aqueous solution as solvent. Thus, large excess of the reagent 1 (at least 6 equivalents) and the use of ethanol-aqueous (v/v = 2:1) solvent was required to obtain the optimum yield (73%) of the desired product 3. After the reaction was completed (2 h), an additional amount of water was added slowly to the reaction mixture until a precipitate occurred. Compound 3 was easily isolated from reaction mixture by vacuum filtration due to its complete insolubility in water. In contrast, compound 4 is slightly soluble in water, and appeared as white amorphous precipitate after remaining one day in the filtrate.
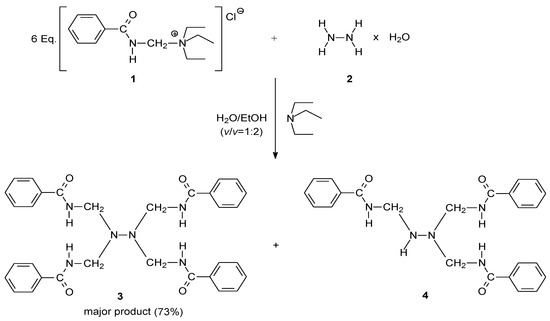
Scheme 1.
Synthetic route to the title compound 3.
The structure of the newly synthesized compound 3 was characterized on the basis of 1H-NMR, 13C-NMR, IR and mass spectral data. The 1H-NMR and 13C-NMR spectra of 3 showed only a few signals, implying the presence of a highly symmetrical compound. Seven of the signals in the 13C-NMR spectrum of 3 were recognized (1 C, 1 CH2, 5 CH), which were assigned to a carbonyl (δ 166.88), a methylene (δ 56.36) and an aromatic group (δ 127.20–134.11), and therefore indicated for the presence of benzamidomethyl group. Moreover, in the 1H-NMR of 3 revealed the absence of the hydrazine protons, which indicates that all of the hydrazine protons have been substituted. Mass spectrometric analysis of compound 3 using ESI technique indicated a molecular ion (m/z = 587.2; [M+Na]+) which is consistent with the molecular formula of a 1,1,2,2-tetrakis[(benzoylamino)methyl]hydrazine (C32H32N6O4, calc. 564). The values are in complete agreement with the structure assigned.
The spectral characterization of 4 was reported by our group previously [22].
3. Experimental
The starting material (benzamidomethyl)triethylammonium chloride 1 was synthesized based on a literature method [21].
3.1. Synthesis of 1,1,2,2-tetrakis[(benzoylamino)methyl]hydrazine (3)
To a solution of (benzamidomethyl)triethylammonium chloride (1.576 g, 5.82 mmol) in 10 mL of water and 0.2 mL of triethylamine, a solution of hydrazine monohydrate (48.56 mg, 0.97 mmol) in 20 mL of ethanol was added. The resulting mixture was stirred for 2 h at room temperature. Afterwards, water was added slowly to the reaction mixture until a precipitate occurred. The product 3 was obtained by vacuum filtration as a white amorphous solid (0.4 g, 73%). The purification was performed by dissolving the product in ethanol and precipitation with water. The same isolation and purification procedure was followed for the formed precipitate 4 in the filtrate from the first filtration step.
Melting point: 99–101 °C (uncorrected);
FTIR (KBr, cm−1): 3303 v(N-H), 1650 (C=O, Amide I), 1533 (N-H, Amide II);
1H-NMR (250 MHz, DMSO-d6): δ/ppm 8.90 (t, J = 5.0 Hz, 1(4)H, CONH), 7.41–7.90 (m, 5(20)H, ArH), 4.55 (d, J = 5.0 Hz, 2(8)H, CH2);
13C NMR (62.9 MHz, CDCl3): δ/ppm 166.88 C=O; 56.36 CH2; Ar: 134.11, 131.43, 128.31, 127.27;
MS (ESI, pos) m/z: 587.2 (M+Na)+.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
We would like to express our gratitude to the Macedonian Ministry of Education and Science (Contract 03-1586) and to the Bulgarian National Science Fund (Contract BM-02/07) for financial support of this research.
References
- Tiwari, K.A.; Singh, K.V.; Bajpai, A.; Shukla, G.; Singh, S.; Mishra, K.A. Synthesis and biological properties of 4-(3H)-quinazolone derivatives. Eur. J. Med. Chem. 2007, 42, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Mai, A.; Artico, M.; Valente, S.; Cerbara, I.; Befani, O.; Turini, P.; Vedova, D.L.; Agostinelli, E. Synthesis and biochemical evaluation of (R)-5-acyloxymethyl- and (S)-5-acylaminomethyl-3-(1H-pyrrol-1-yl)-2-oxazolidinones as new anti-monoamine oxidase (anti-MAO) agents. ARKIVOC 2004, 2004, 32–43. [Google Scholar]
- Zlotin, G.S.; Sharova, V.I.; Luk`yanov, A.O. Chemical properties of N-(amidomethy)- and N-(imidomethyl)glycine derivatives 2. Reactions at alkoxycarbonyl and carboxyl groups. Rus. Chem. Bull. 1996, 45, 1680–1687. [Google Scholar] [CrossRef]
- Schioppacassi, G.; Morvillo, E.; Bruna, C.D.; Franceschi, G.; Foglio, M. In vitro and in vivo evaluation of benzamidomethyl-benzylpenicillinate (FI7303). A new 'repository' form. Chemotherapy 1978, 24, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; Nielsen, N.M.; Buur, A. Aspirin prodrugs: Synthesis and hydrolysis of 2-acetoxybenzoate esters of various N-(hydroxyalkyl) amides. Int. J. Pharm. 1988, 44, 151–158. [Google Scholar] [CrossRef]
- Moreira, R.; Calheiros, T.; Cabrita, J.; Mendes, E.; Pimentel, M.; Iley, J. Acyloxymethyl as a drug protecting group. Part 3. Tertiary O-Amidomethyl esters of penicillin G: Chemical hydrolysis and anti-bacterial activity. Pharm. Res. 1996, 13, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Getz, J.J.; Prankerd, R.J.; Sloan, K.B. Mechanism of hydrolysis of benzamidomethyl derivatives of phenols and its implications for prodrug design. J. Org. Chem. 1992, 57, 1702–1706. [Google Scholar] [CrossRef]
- Ragnarsson, U. Synthetic methodology for alkyl substituted hydrazines. Chem. Soc. Rev. 2001, 30, 205–213. [Google Scholar] [CrossRef]
- Ling, L.; Urichuk, L.J.; Sloley, B.D.; Coutts, R.T.; Baker, G.B.; Shan, J.J.; Pang, P.K.T. Synthesis of N-propargylphenelzine and analogues as neuroprotective agents. Bioorg. Med. Chem. Lett. 2001, 11, 2715–2717. [Google Scholar] [CrossRef]
- Rabong, C.; Jordis, U.; Phopase, J.B. NXO Building Blocks for Backbone Modification of Peptides and Preparation of Pseudopeptides. J. Org. Chem. 2010, 75, 2492–2500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Durkin, J.P.; Windsor, W.T. Azapeptides as inhibitors of the hepatitis C virus NS3 serine protease. Bioorg. Med. Chem. Lett. 2002, 12, 1005–1008. [Google Scholar] [CrossRef]
- Raja, A.; Lebbos, J.; Kirkpatrick, P. Fresh from the Pipeline: Atazanavir sulphate. Nat. Rev. Drug Discov. 2003, 2, 857–858. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Cherney, M.M.; Huitema, C.; Liu, J.; James, K.E.; Powers, J.C.; Eltis, L.D.; James, M.N.G. Crystal structures of the main peptidase from the SARS coronavirus inhibited by a substrate-like aza-peptide epoxide. J. Mol. Biol. 2005, 353, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Clennan, E.L.; Noe, L.J.; Wen, T. Hydrazines: New charge-transfer physical quenchers of singlet oxygen. J. Am. Chem. Soc. 1990, 112, 5080–5085. [Google Scholar] [CrossRef]
- Nelsen, S.F.; Rumack, D.T.; Meot-Ner, M. Kinetic effects of an unusually large neutral to radical cation geometry change. Slow electron-transfer reactions between alkylhydrazines. J. Am. Chem. Soc. 1987, 109, 1373–1379. [Google Scholar] [CrossRef]
- Nelsen, S.F.; Rumack, D.T.; Meot-Ner, M. Gas-phase electron-transfer equilibrium studies on tetraalkylhydrazines: Geometry effects on ionization thermochemistry, relaxation energies, and ion solvation energies. J. Am. Chem. Soc. 1988, 110, 7945–7952. [Google Scholar] [CrossRef]
- Nelsen, S.F.; Peacock, V.; Weisman, G.R. Single-electron oxidation equilibriums of tetraalkylhydrazines. Comparison of solution E0 values and vapor-phase ionization potentials. J. Am. Chem. Soc. 1976, 98, 5269–5277. [Google Scholar] [CrossRef]
- Nelsen, S.F.; Hintz, P.J. Electrochemical oxidation of tetraalkylhydrazines. Effects of hydrazine and hydrazine radical cation geometry. J. Am. Chem. Soc. 1972, 94, 7108–7113. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Benzamidomethylation with (benzamidomethyl)triethylammonium chloride. 2. A simple method for benzamidomethylation of thiols, amines and carboxylic acods. Molecules 2000, 5, 927–936. [Google Scholar] [CrossRef]
- Popovski, E. Synthesis of N-(N’-benoylhydrazinomethyl)benzamide. Molbank 2007, 2007, M525. [Google Scholar] [CrossRef]
- Popovski, E. Synthesis of N-[N’-(2-hydroxy-2,2-diphenylacethyl)hydrazinomethyl]benzamide. Molbank 2007, 2007, M526. [Google Scholar] [CrossRef]
- Tanatarec, J.; Mikova, B.; Popovski, E. Tribenzamidomethyl hydrazine. Molbank 2010, 2010, M710. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Simple Method for Benzamidomethylation of Phenols in Water Solution. Synth. Commun. 1999, 29, 3451–3458. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).