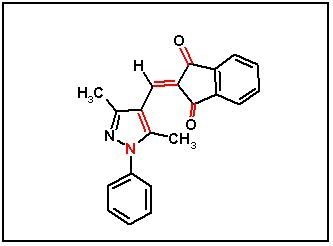
2-[(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)methylene]indane-1,3-dione
Abstract
: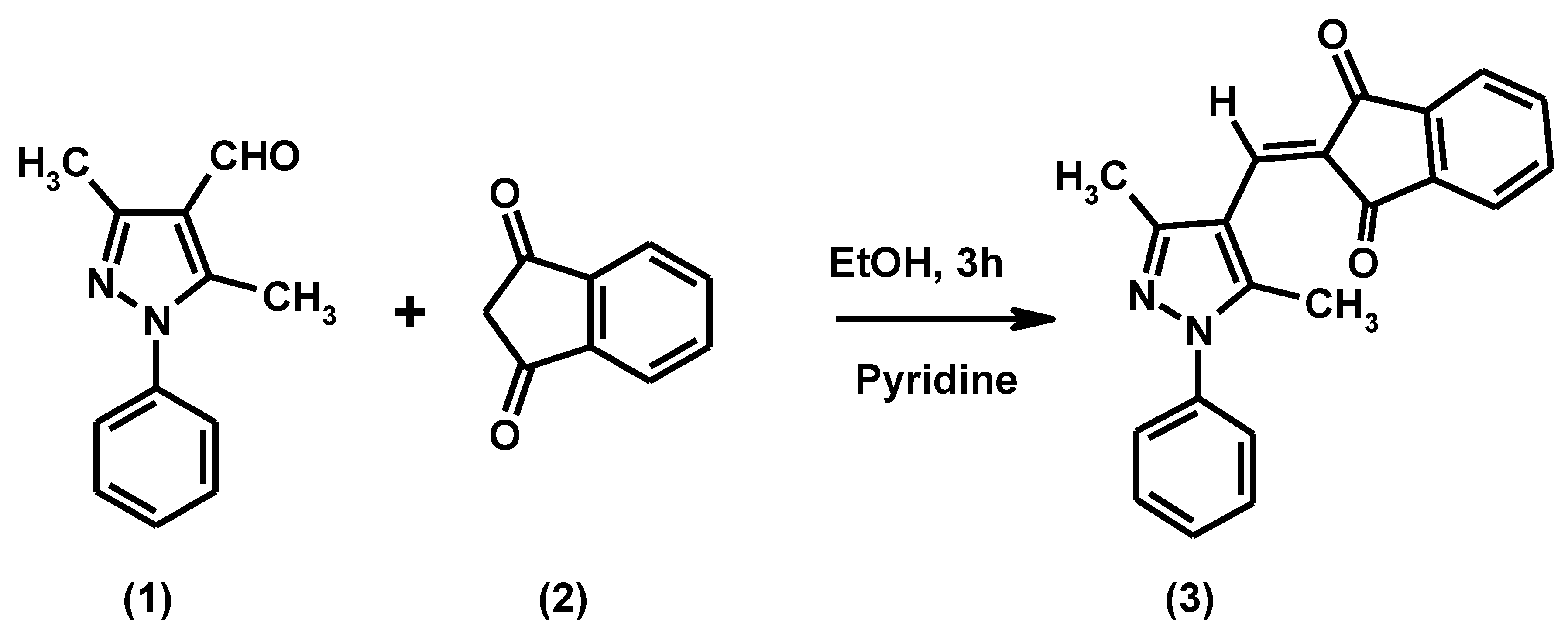
Experimental
Materials
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Mitchell, R.E.; Greenwood, D.R.; Sarojini, V. An antibacterial pyrazole derivative from Burkholderia glumae, a bacterial pathogen of rice. Phytochemistry 2008, 69, 2704–2707. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolotti, O.; Carotti, A. Design, synthesis and pharmacobiological evaluation of novel acrylic acid derivatives acting as lipoxygenase and cyclooxygenase-1 inhibitors with antioxidant and anti-inflammatory activities. Eur. J. Med. Chem. 2011, 46, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, M.; Loizzo, M.R.; Statti, G.A.; Michel, S.; Tillequin, F.; Menichini, F. The synthesis and Angiotensin Converting Enzyme (ACE) inhibitory activity of chalcones and their pyrazole derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 1990–1993. [Google Scholar] [CrossRef] [PubMed]
- Bonacorso, H.G.; Navarini, J.; Wiethan, C.W.; Bortolotto, G.P.; Paim, G.R.; Cavinatto, S.; Martins, M.A.P.; Zanatta, N.; Caro, M.S.B. The first application of 4-alkoxy-1,1,1-trifluoroalk-3-en-2-ones in a three-component condensation protocol for the synthesis of 3-acyl-4-aryl-2 (trifluoromethyl)-2-hydroxy-3,4,7,8-tetrahydro-2H-chromen-5(6H)-ones. J. Fluorine Chem. 2011, in press. [Google Scholar] [CrossRef]
- Sadr, M.H.; Sardroodi, J.J.; Zare, D.; Brooks, N.R.; Clegg, W.; Song, Y. Nonlinear optical properties and crystal structure determination of a pentanuclear copper(I) cluster (NEt4)2[MoS4(CuBp)4] (Bp = H2B(pyrazolyl)2). Polyhedron 2006, 25, 3285–3288. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Cui, Y.-P. The synthesis, structure and spectroscopic properties of novel oxazolone-, pyrazolone- and pyrazoline-containing heterocycle chromophores. Dye. Pigment. 2009, 81, 27–34. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, K.; Gong, F.; Li, S.; Chen, J.; Ma, J.S.; Sobenina, L.N.; Mikhaleva, A.I.; Trofimov, B.A.; Yang, G. A highly selective fluorescent sensor for fluoride anion based on pyrazole derivative: Naked eye “no–yes” detection. J. Photochem. Photobiol. A Chem. 2011, 217, 29–34. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Li, Y.; Li, S.; Wang, L. A study of the inhibition of iron corrosion by imidazole and its derivatives self-assembled films. Corros. Sci. 2009, 51, 291–300. [Google Scholar] [CrossRef]
- Asiri, A.M.; Khan, S.A. 2,6-Bis(9-ethyl-9H-carbazolylmethylene)cyclohexanone. Molbank 2009, 2009, M635. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asiri, A.M.; Khan, S.A. 2-[(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)methylene]indane-1,3-dione. Molbank 2011, 2011, M720. https://doi.org/10.3390/M720
Asiri AM, Khan SA. 2-[(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)methylene]indane-1,3-dione. Molbank. 2011; 2011(1):M720. https://doi.org/10.3390/M720
Chicago/Turabian StyleAsiri, Abdullah M., and Salman A. Khan. 2011. "2-[(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)methylene]indane-1,3-dione" Molbank 2011, no. 1: M720. https://doi.org/10.3390/M720
APA StyleAsiri, A. M., & Khan, S. A. (2011). 2-[(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)methylene]indane-1,3-dione. Molbank, 2011(1), M720. https://doi.org/10.3390/M720