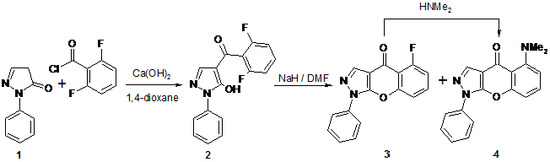
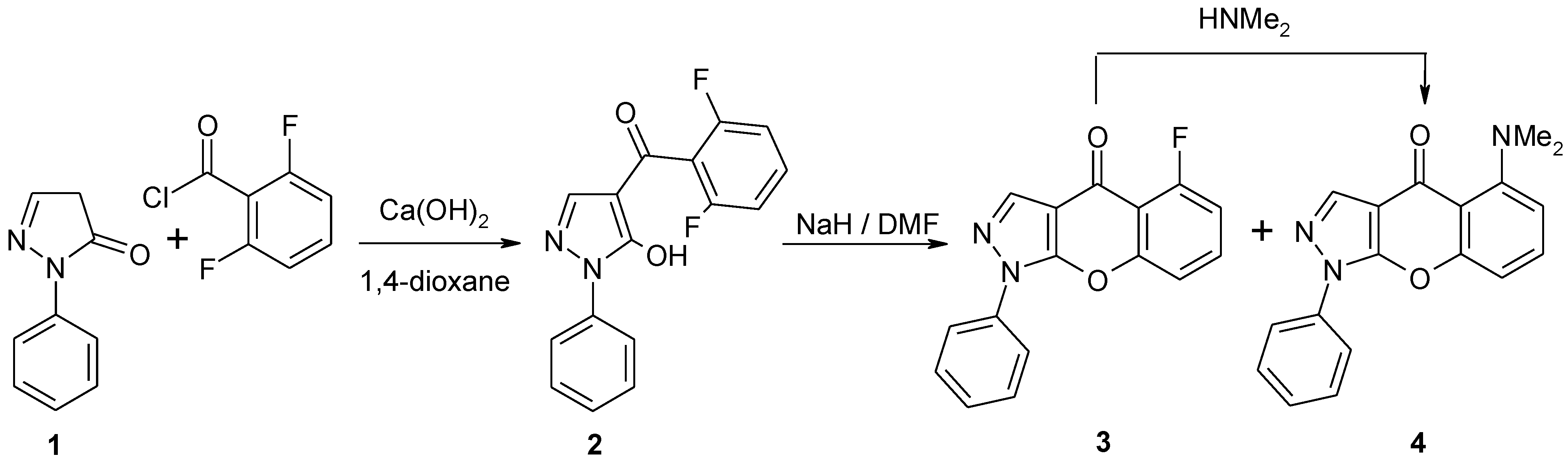
5-Dimethylamino-1-phenylchromeno[2,3-c]pyrazol-4(1H)-one
Abstract
:Experimental
5-Dimethylamino-1-phenylchromeno[2,3-c]pyrazol-4(1H)-one (4)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Eller, G.A.; Wimmer, V.; Haring, A.W.; Holzer, W. An Efficient Approach to Heterocyclic Analogues of Xanthone: A Short Synthesis of All Possible Pyrido[5,6]pyrano[2,3-c]pyrazol-4(1H)-ones. Synthesis 2006, 4219–4229. [Google Scholar] [CrossRef]
- Eller, G.A.; Haring, A.W.; Datterl, B.; Zwettler, M.; Holzer, W. Tri- and Tetracyclic Heteroaromatic Systems: Synthesis of Novel Benzo-, Benzothieno- and Thieno-Fused Pyrano[2,3-c]pyrazol-4(1H)-ones. Heterocycles 2007, 71, 87–104. [Google Scholar]
- Eller, G.A.; Holzer, W. A Convenient Approach to Heterocyclic Building Blocks: Synthesis of Novel Ring Systems Containing a [5,6]Pyrano[2,3-c]pyrazol-4(1H)-one Moiety. Molecules 2007, 12, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Eller, G.A.; Datterl, B.; Holzer, W. Pyrazolo[4’,3’:5,6]pyrano[2,3-b]quinoxalin-4(1H)-one: Synthesis and Characterization of a Novel Tetracyclic Ring System. J. Heterocycl. Chem. 2007, 44, 1139–1143. [Google Scholar] [CrossRef]
- Eller, G.A.; Wimmer, V.; Holzer, W. Synthesis of Novel Polycyclic Ring Systems Containing Two Pyrano[2,3-c]pyrazol-4(1H)-one Moieties. Khim. Geterotsikl. Soedin. 2007, 1251–1255, Chem. Heterocycl. Comp. 2007, 43, 1060–1064. [Google Scholar]
- Eller, G.A.; Habicht, D.; Holzer, W. Synthesis of a Novel Pentacycle: 8-Methyl-10-phenylpyrazolo[4’,3’:5,6]pyrano[3,2-c][1,10]phenanthrolin-7(10H)-one. Khim. Geterotsikl. Soedin. 2008, 884–890, Chem. Heterocycl. Comp. 2008, 44, 709–714. [Google Scholar]
- Eller, G.A.; Zhang, Q.; Habicht, D.; Datterl, B.; Holzer, W. Synthesis and NMR Data of Pyrazolo[4’,3’:5,6]pyrano[2,3-b]pyrazin-4(1H)-ones: Derivatives of a Novel Tricyclic Ring System. Acta Chim. Slov. 2009, 56, 521–526. [Google Scholar]
- Böhme, H.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. Fluorine in Medicinal Chemistry. ChemBioChem 2004, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, W.K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Sharon, A.; Chu, C.K. Fluorinated nucleosides: Synthesis and biological implication. J. Fluorine Chem. 2008, 129, 129743–129766. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, D.B.; Karukurichi, K.R.; de la Salud-Bea, R.; Nelson, D.L.; McCune, C.D. Use of fluorinated functionality in enzyme inhibitor development: Mechanistic and analytical advantages. J. Fluorine Chem. 2008, 129, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I. Fluorine in Medicinal Chemistry And Chemical Biology; Wiley: Chichester, UK, 2009; Chem. Abstr. 2009, 152, 399832. [Google Scholar]
- Holzer, W.; Ebner, A.; Schalle, K.; Batezila, G.; Eller, G.A. Novel fluoro-substituted benzo- and benzothieno fused pyrano[2,3-c]pyrazol-4(1H)-ones. J. Fluorine Chem. 2010, 131, 1013–1024. [Google Scholar] [CrossRef]
- Jensen, B.S. The Synthesis of 1-Phenyl-3-methyl-4-acyl-pyrazolones-5. Acta Chem. Scand. 1959, 13, 1668–1670, Chem. Abstr. 1962, 56, 66890. [Google Scholar]
- Perrin, D.D.; Armarego, W.L.F.; Perrin, D.R. Purification of Laboratory Chemicals, 2nd ed.; Pergamon Press: Oxford, UK, 1980; p. 224. [Google Scholar]
- Lee, W.S.; Yoon, Y.-J.; Kim, S.-K. Reaction of Chloropyridazines with N,N- Dimethylformamide. J. Heterocycl. Chem. 2000, 37, 1591–1595. [Google Scholar] [CrossRef]
- Braun, S.; Kalinowski, H.-O.; Berger, S. 150 and More Basic NMR Experiments: A Practical Course – Second Expanded Edition; Wiley–VCH: Weinheim, Germany, 1998; Chem. Abstr. 1999, 131, 184497. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ebner, A.; Holzer, W. 5-Dimethylamino-1-phenylchromeno[2,3-c]pyrazol-4(1H)-one. Molbank 2010, 2010, M706. https://doi.org/10.3390/M706
Ebner A, Holzer W. 5-Dimethylamino-1-phenylchromeno[2,3-c]pyrazol-4(1H)-one. Molbank. 2010; 2010(4):M706. https://doi.org/10.3390/M706
Chicago/Turabian StyleEbner, Angelika, and Wolfgang Holzer. 2010. "5-Dimethylamino-1-phenylchromeno[2,3-c]pyrazol-4(1H)-one" Molbank 2010, no. 4: M706. https://doi.org/10.3390/M706
APA StyleEbner, A., & Holzer, W. (2010). 5-Dimethylamino-1-phenylchromeno[2,3-c]pyrazol-4(1H)-one. Molbank, 2010(4), M706. https://doi.org/10.3390/M706