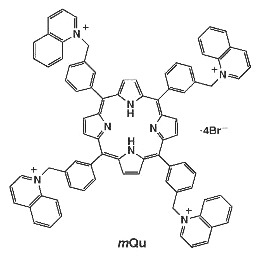
1,1’,1’’,1’’’-[Porphyrin-5,10,15,20-tetrayltetrakis(3,1-phenylenemethylene)]tetraquinolinium Tetrabromide
Abstract
:
Experimental
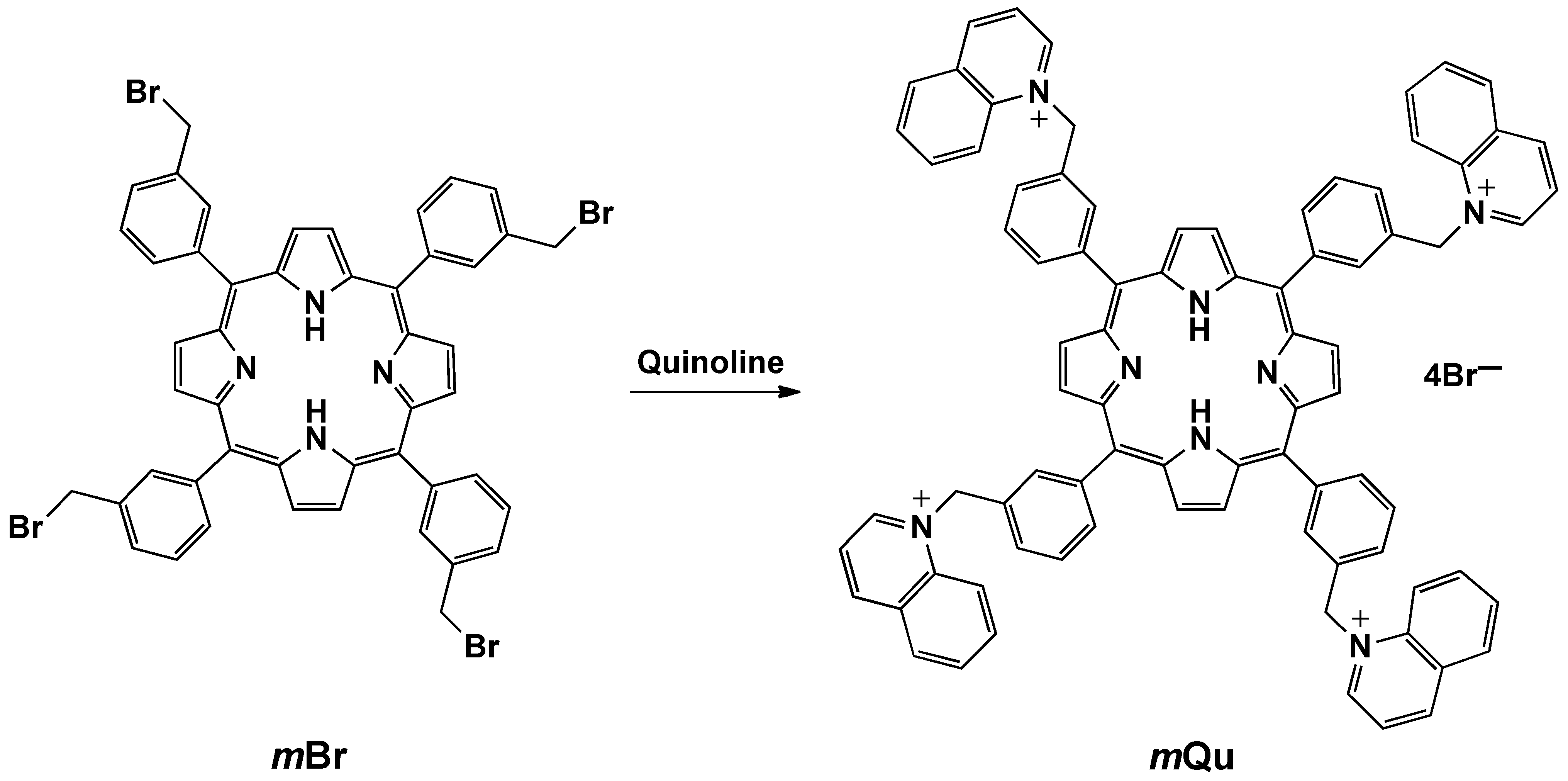
1,1’,1’’,1’’’-[Porphyrin-5,10,15,20-tetrayltetrakis(3,1-phenylenemethylene)]tetraquinolinium tetrabromide (mQu)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Han, F.X.G.; Wheelhouse, R.T.; Hurley, L.H. Interactions of TMPyP4 and TMPyP2 with quadruplex DNA. Structural basis for the differential effects on telomerase inhibition. J. Am. Chem. Soc. 1999, 121, 3561–3570. [Google Scholar] [CrossRef]
- Izbicka, E.; Wheelhouse, R.T.; Raymond, E.; Davidson, K.K.; Lawrence, R.A.; Sun, D.Y.; Windle, B.E.; Hurley, L.H.; Von Hoff, D.D. Effects of cationic porphyrins as G-quadruplex interactive agents in human tumor cells. Cancer Res. 1999, 59, 639–644. [Google Scholar] [PubMed]
- De Cian, A.; Cristofari, G.; Reichenbach, P.; De Lemos, E.; Monchaud, D.; Teulade-Fichou, M.P.; Shin-Ya, K.; Lacroix, L.; Lingner, J.; Mergny, J.L. Reevaluation of telomerase inhibition by quadruplex ligands and their mechanisms of action. Proc. Natl. Acad. Sci. USA 2007, 104, 17347–17352. [Google Scholar] [CrossRef] [PubMed]
- Mikami-Terao, Y.; Akiyama, M.; Yuza, Y.; Yanagisawa, T.; Yamada, O.; Kawano, T.; Agawa, M.; Ida, H.; Yamada, H. Antitumor activity of TMPyP4 interacting G-quadruplex in retinoblastoma cell lines. Exp. Eye. Res. 2009, 89, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Uno, T.; Ishikawa, Y. Stabilization of guanine quadruplex DNA by the binding of porphyrins with cationic side arms. Bioorg. Med. Chem. 2005, 13, 2423–2430. [Google Scholar] [CrossRef] [PubMed]
- Bookser, B.C.; Bruice, T.C. Syntheses of quadruply two- and three-atom, aza-bridged, cofacial bis(5,10,15,20-tetraphenylporphyrins). J. Am. Chem. Soc. 1991, 113, 4208–4218. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ishikawa, Y.; Yamashita, T.; Fujii, S.; Uno, T. 1,1’,1’’,1’’’-[Porphyrin-5,10,15,20-tetrayltetrakis(3,1-phenylenemethylene)]tetraquinolinium Tetrabromide. Molbank 2010, 2010, M704. https://doi.org/10.3390/M704
Ishikawa Y, Yamashita T, Fujii S, Uno T. 1,1’,1’’,1’’’-[Porphyrin-5,10,15,20-tetrayltetrakis(3,1-phenylenemethylene)]tetraquinolinium Tetrabromide. Molbank. 2010; 2010(4):M704. https://doi.org/10.3390/M704
Chicago/Turabian StyleIshikawa, Yoshinobu, Takeshi Yamashita, Satoshi Fujii, and Tadayuki Uno. 2010. "1,1’,1’’,1’’’-[Porphyrin-5,10,15,20-tetrayltetrakis(3,1-phenylenemethylene)]tetraquinolinium Tetrabromide" Molbank 2010, no. 4: M704. https://doi.org/10.3390/M704
APA StyleIshikawa, Y., Yamashita, T., Fujii, S., & Uno, T. (2010). 1,1’,1’’,1’’’-[Porphyrin-5,10,15,20-tetrayltetrakis(3,1-phenylenemethylene)]tetraquinolinium Tetrabromide. Molbank, 2010(4), M704. https://doi.org/10.3390/M704