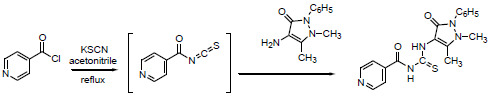
N-[(2,5-Dimethyl-3-oxo-1-phenyl-2,3-dihydro-1H-pyrazol-4-yl)carbamothioyl]isonicotinamide
Abstract
:1. Introduction
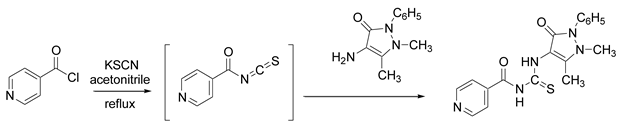
2. Experimental
2.1. General
2.2. N-[(2,5-dimethyl-3-oxo-1-phenyl-2,3-dihydro-1H-pyrazol-4-yl)carbamothioyl]isonicotinamide
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References
- Neucki, E. Zur Kenntniss des Sulfoharnstoffs. Ber. Dtsch. Chem. Ges. 1873, 6, 598–600. [Google Scholar] [CrossRef]
- Douglass, I.B.; Dains, F.B. Some Derivatives of Benzoyl and Furoyl Isothiocyanates and their Use in Synthesizing Heterocyclic Compounds. J. Am. Chem. Soc. 1934, 56, 719–721. [Google Scholar] [CrossRef]
- Koch, K.R. New Chemistry with Old Ligands: N-Alkyl- and N,N-Dialkyl-N′-acyl(aroyl) thioureas in Co-ordination, Analytical and Process Chemistry of the Platinum Group Metals. Coordin. Chem. Rev. 2001, 216-217, 473–488. [Google Scholar] [CrossRef]
- Sandor, M.; Geistmann, F.; Schuster, M. An Anthracene-substituted Benzoylthioure for the Selective Determination of Hg(II) in Micellar Media. Anal. Chim. Acta 1999, 388, 19–26. [Google Scholar] [CrossRef]
- Del Campo, R.; Criado, J.J.; Garcia, E.; Hermosa, M.R.; Jimenez-Sanchez, A.; Manzano, J.L.; Monte, E.; Rodriguez, E.; Sanz, F. Thiourea Derivatives and Their Nickel(II) and Platinum(II) Complexes: Antifungal Activity. J. Inorg. Biochem. 2002, 89, 74–82. [Google Scholar] [CrossRef]
- Xu, X.; Qian, X.; Li, Z.; Huang, Q.; Chen, G. Synthesis and Insecticidal Activity of New Substituted N-Aryl-N′-benzoylthiourea Compounds. J. Fluorine Chem. 2003, 121, 51–54. [Google Scholar] [CrossRef]
- Bondock, S.; Rabie, R.; Etman, H.A.; Fadda, A.A. Synthesis and Antimicrobial Activity of Some New Heterocycles, Incorporating Antipyrine Moiety. Eur. J. Med. Chem. 2008, 43, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aydin, F.; Dağci, E. N-[(2,5-Dimethyl-3-oxo-1-phenyl-2,3-dihydro-1H-pyrazol-4-yl)carbamothioyl]isonicotinamide. Molbank 2010, 2010, M682. https://doi.org/10.3390/M682
Aydin F, Dağci E. N-[(2,5-Dimethyl-3-oxo-1-phenyl-2,3-dihydro-1H-pyrazol-4-yl)carbamothioyl]isonicotinamide. Molbank. 2010; 2010(2):M682. https://doi.org/10.3390/M682
Chicago/Turabian StyleAydin, Fatma, and Erdoğan Dağci. 2010. "N-[(2,5-Dimethyl-3-oxo-1-phenyl-2,3-dihydro-1H-pyrazol-4-yl)carbamothioyl]isonicotinamide" Molbank 2010, no. 2: M682. https://doi.org/10.3390/M682
APA StyleAydin, F., & Dağci, E. (2010). N-[(2,5-Dimethyl-3-oxo-1-phenyl-2,3-dihydro-1H-pyrazol-4-yl)carbamothioyl]isonicotinamide. Molbank, 2010(2), M682. https://doi.org/10.3390/M682