Synthesis of New Schiff Base Ether: p-n-(Dimethylamino)-benzylidene-p-dodecyloxyaniline
Abstract
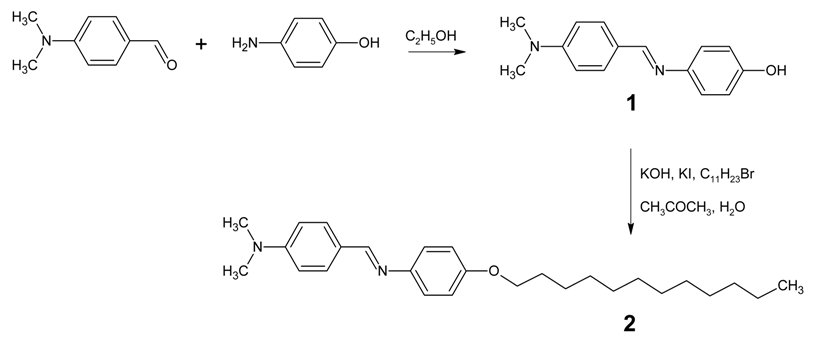
:
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Jacobi, A.; Weissflog, W. Liq. Cryst. 1997, 22, 107. [CrossRef]
- Yeap, G.Y.; Ha, S.T.; Lim, P.L.; Boey, P.L.; Mahmood, W.A.K.; Ito, M.M.; Sanehisa, S. Mol. Cryst. Liq. Cryst. 2004, 423, 73–84.
- Yeap, G.Y.; Ha, S.T.; Lim, P.L.; Boey, P.L.; Ito, M.M.; Sanehisa, S.; Vill, V. Mol. Cryst. Liq. Cryst. 2006, 452, 63–72. [CrossRef]
- Yeap, G.Y.; Ha, S.T.; Boey, P.L.; Mahmood, W.A.K.; Ito, M.M.; Youhei, Y. Mol. Cryst. Liq. Cryst. 2006, 452, 73–90. [CrossRef]
- Yeap, G.Y.; Ha, S.T.; Lim, P.L.; Boey, P.L.; Ito, M.M.; Sanehisa, S.; Youhei, Y. Liq. Cryst. 2006, 33, 205–211. [CrossRef]
- Ha, S.T.; Ong, L.K.; Win, Y.F.; Koh, T.M.; Yeap, G.Y. Molbank 2008, 3, M582. [CrossRef]
- Ha, S.T.; Ong, L.K.; Win, Y.F.; Koh, T.M.; Yeap, G.Y. Molbank 2009, 1, M584.
- Ha, S.T.; Ong, L.K.; Win, Y.F.; Koh, T.M.; Yeap, G.Y. Molbank 2009, 1, M585.
- Ha, S.T.; Ong, L.K.; Win, Y.F.; Koh, T.M.; Yeap, G.Y. Molbank 2009, 1, M591.
- Narasimhaswamy, T.; Srinivasan, K.S.V. Liq. Cryst. 2004, 31, 1457–1462. [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ha, S.-T.; Ong, L.-K.; Wong, J.P.-W.; Win, Y.-F.; Koh, T.-M. Synthesis of New Schiff Base Ether: p-n-(Dimethylamino)-benzylidene-p-dodecyloxyaniline. Molbank 2009, 2009, M598. https://doi.org/10.3390/M598
Ha S-T, Ong L-K, Wong JP-W, Win Y-F, Koh T-M. Synthesis of New Schiff Base Ether: p-n-(Dimethylamino)-benzylidene-p-dodecyloxyaniline. Molbank. 2009; 2009(1):M598. https://doi.org/10.3390/M598
Chicago/Turabian StyleHa, Sie-Tiong, Lay-Khoon Ong, Joanna Pik-Wan Wong, Yip-Foo Win, and Teck-Ming Koh. 2009. "Synthesis of New Schiff Base Ether: p-n-(Dimethylamino)-benzylidene-p-dodecyloxyaniline" Molbank 2009, no. 1: M598. https://doi.org/10.3390/M598
APA StyleHa, S.-T., Ong, L.-K., Wong, J. P.-W., Win, Y.-F., & Koh, T.-M. (2009). Synthesis of New Schiff Base Ether: p-n-(Dimethylamino)-benzylidene-p-dodecyloxyaniline. Molbank, 2009(1), M598. https://doi.org/10.3390/M598



