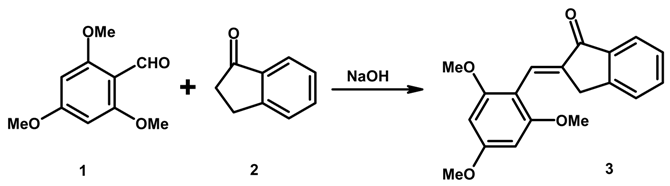
Synthesis of (2E)-2-(2,4,6-Trimethoxybenzylidene)indan-1-one
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Fabian, J.; Hartmann, H. Light Absorption of Organic Colorants; Springer Verlag: Berlin, 1980. [Google Scholar]
- Asiri, A. M. Synthesis and characterization of dyes exemplified by 2-arylidene-1-dicyanomethyleneindane. Dyes and Pigments 1999, 42, 209. [Google Scholar] [CrossRef]
- Attia, A.; Michael, M. Azachalcones. Part 2: Reactions of 3,3-diazachalcones. Pharmazie 1982, 37, 551. [Google Scholar] [PubMed]
- Asiri, A. M. Synthesis and Absorption Spectral Properties of Bis-methine Dyes Exemplified by 2,5-Bis-arylidene-1-dicyanomethylene-cyclopentanes. Bull. Korean Chem. Soc. 2003, 24, 426. [Google Scholar] [CrossRef] [Green Version]
- The title compound was obtained as a single isomer, accotrding to TLC; assignment of the E configuration is based on the chemical shift (7.83 ppm) of the olefinic proton.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asiri, A.M.; Rasul, M.G. Synthesis of (2E)-2-(2,4,6-Trimethoxybenzylidene)indan-1-one. Molbank 2009, 2009, M588. https://doi.org/10.3390/M588
Asiri AM, Rasul MG. Synthesis of (2E)-2-(2,4,6-Trimethoxybenzylidene)indan-1-one. Molbank. 2009; 2009(1):M588. https://doi.org/10.3390/M588
Chicago/Turabian StyleAsiri, Abdullah Mohamed, and Mohammed Golam Rasul. 2009. "Synthesis of (2E)-2-(2,4,6-Trimethoxybenzylidene)indan-1-one" Molbank 2009, no. 1: M588. https://doi.org/10.3390/M588
APA StyleAsiri, A. M., & Rasul, M. G. (2009). Synthesis of (2E)-2-(2,4,6-Trimethoxybenzylidene)indan-1-one. Molbank, 2009(1), M588. https://doi.org/10.3390/M588



