Abstract
In this study, a fast and good yield one-pot microwave-assisted synthesis (45 seconds) of 1H-phenanthro[9,10-d][1,2,3]triazole by a 1,3-dipolar cycloaddition reaction of sodium azide and 9-bromophenanthrene in the presence of potassium tert-butoxide in DMSO as solvent is reported.
Keywords:
Microwave assisted synthesis; 1; 3-Dipolar cycloadditions; Benzyne intermediate; 1H-Phenanthro[9; 10-d][1; 2; 3]triazole; 1; 2; 3-Triazoles 1. Introduction
Microwave-assisted synthesis has been utilized as a powerful and effective technique to promote a group of chemical reactions [1,2,3,4,5,6,7,8]. Huisgen’s 1,3-dipolar cycloaddition of alkynes and azides yielding triazoles is, undoubtedly, the premier example of click chemistry reactions [9,10,11,12,13,14,15,16,17,18,19,20,21,22]. 1,2,3-Triazoles are known to be relatively resilient to metabolic degradation and have been known utility in several medicinal chemistry campaigns as isosteres for phenyl rings and carboxyl functionalities [17]. The triazoles may display a wide range of biological activity as anti-HIV and anti-microbial agents as well as selective β3 adrenergic receptor agonist and anti allergic agents [18,19,20,21,22]. Additionally, 1,2,3-triazoles are found in herbicides, fungicides and dyes [12,23]. Due to these interesting activities, fast and new methods for the synthesis of these compounds should be significant. In general, 1,2,3-triazole formation requires harsh conditions, that is, high temperature and longer reaction times. In the original description, the explored examples showed that although these were relatively clean processes, they could take from 12 to 48 hours at high temperatures (~110 °C) [17]. The mechanistic proposal of the Cu(I)-catalyzed alkyne-azide 1,3- dipolar cycloaddition was reported and found to involve polar transition states, favorable for microwave activation [29,30]. The Huisgen 1,3-dipolar cycloaddition of azides and alkynes resulting in 1,2,3-triazoles is one of the most powerful click reactions [12,23,24,25,26,27,28]. An example of microwave-assisted azide-alkyne cycloaddition was reported by Katritzky et al. [9]. The reactions have involved primary azides and acetylenic amide. Recently, the synthesis of simple alkyloxycarbonyl-1,2,3-triazole derivatives were reported by reaction between N3¯ and alkylpropiolates [18,29,30,31,32]. The total reaction time was 48 hours [31,32].
Little attention has been given to the chemistry and the applications of 1H-Phenanthro[9,10-d]- [1,2,3]triazole (1). This compound and its derivatives are widely used as anti-fog, reagents in the photo- industry, inhibitors for corrosion of metals, and ultraviolet absorbers. The reported method for the synthesis of 1 requires more than 12 hours of total reaction time [33].
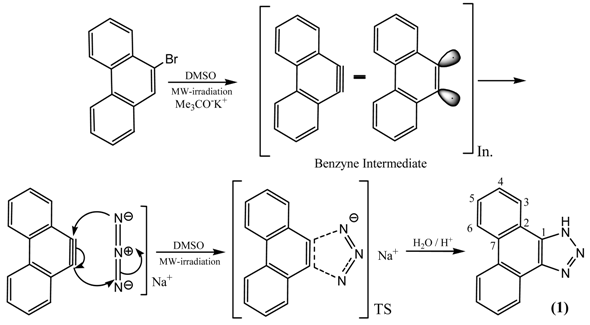
Herein we report a fast one-pot microwave-assisted synthesis of 1H-phenanthro[9,10-d][1,2,3]triazole (1) by 1,3-dipolar cycloaddition reaction of sodium azide (NaN3) and 9-bromophenanthrene in DMSO as solvent, affording the title compound in good yields. It is proposed that a benzyne intermediate is generated from 9-bromophenanthrene under the reaction conditions, which undergoes cycloaddition with azide [33].
2. Experiment and Results
2.1. 1H-Phenanthro[9,10-d][1,2,3]triazole (1)
A mixture of 9-bromophenanthrene (0.5 g, 0.002 mol), potassium tert-butoxide (0.9 g, 0.008 mol) and sodium azide (NaN3) (0.3 g, 0.005 mol) in 5 ml DMSO was made in a dried, heavy-walled Pyrex tube. The vessel was placed in a microwave oven. After 45 seconds irradiation at 700 W power, the mixture was cooled to room temperature. The solvent was evaporated under mild heat and reduced pressure. A solution of HCl (20 ml of the solution H2O, 20 ml + HCl, 7.5 ml, 1 M) was added to the residue. An off- white solid was obtained. The product can be washed with chloroform, acetone and distilled water. This stage gave 0.27 g product (workup yield 62%, lit. 60% [27]). The FT-IR, 1H NMR, 13C NMR, MS (and CI-MS) spectra and CHN analysis of the product 1 were obtained. No details are given about the by- products and only the final products are considered.
Mp 306 ˚C (lit. 306 ˚C [33] and 294-298 ˚C [34]).
FT-IR (KBr): 3273 (N-H), 3225, 1809-1991 (aromatic), 1638, 1471 (C=C, str.), 1452, 1314 (asym. and sym. of –N3), 952, 919, 762 cm-1.
1H NMR δH (DMSO-d6): 8.83 (dd, AX system, 8.6 Hz, 1H, H-3), 8.54 (dd, AX system, 8.6 Hz, 1H, H-6) and 7.74-7.79 (m, 2H, H-4 and H-5).
13C NMR (DMSO-d6): 136.3, 129.6, 128.3, 127.9, 124.6, 123.4 and 123.2.
MS: m/z (relative intensity) 219 (M+, 100%), 190 (45), 164 (40), 110 (21), 78 (53) and 63 (80).
C14H9N3, CHN-analysis; calculated: C, 76.70%; H, 4.14%; N, 19.17%. Found: C, 76.38%; H, 4.16%; N, 19.4%.
Caution: For safety reasons all of the experiments should be performed in an efficient hood in order to avoid contact with vapors, as some quantity of substances can be vaporized during irradiation.
3. Conclusion
The simple one-pot microwave-assisted synthesis of the useful 1H-phenanthro[9,10-d][1,2,3]triazole (1) by a Huisgen 1,3-dipolar cycloaddition reaction of sodium azide (NaN3) and 9-bromophenanthrene in the presence of potassium tert-butoxide (Me3CO−K+) in DMSO as solvent was carried out in good yield. The reaction time was 45 seconds. It is proposed that for this reaction a benzyne intermediate is produced from 9-bromophenanthrene under the conditions. Comparison of this procedure with other methods confirmed the facility and rapidity of this method for the synthesis of 1 as an important compound.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
The author gratefully acknowledges Professor Curt Wentrup and colleagues in the Chemistry Department of The University of Queensland-Australia for their useful suggestions.The authors are grateful to the Research Council of Science and Research Campus and Arak branch of I.A.U for support of this study.
References
- Stadler, A.C.; Kappe, O. Microwave Assisted Org. Synth. 2005, 177–221.
- Kappe, C.O. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [CrossRef] [PubMed]
- Kappe, C.O.; Stadler, A. Microwaves in Organic and Medicinal Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2005. [Google Scholar]
- Zbancioc, N.G.; Caprosu, D.M.; Moldoveanu, C.C.; Ionel, I.I. Arkivoc 2005, 189–198.
- Katritzky, A.R.; Singh, S.K. Arkivoc 2003, 68–86.
- Ling, M.J.; Sun, C. M. Synlett 2004, 4, 663–666.
- Dai, W.-M.; Guo, D.-S.; Sun, L.-P.; Huang, X.-H. Org. Lett. 2003, 5, 2919–2922.
- Finaru, A.; Berthault, A.; Besson, T.; Guillaument, G.; Berteina-Raboin, S. Org. Lett. 2002, 4, 2613–2615.
- Katritzky, A.R.; Singh, S.K. J. Org. Chem. 2002, 67, 9077–9079. [CrossRef]
- Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Angew. Chem. Int. Ed. 2005, 44, 5188–5240. [CrossRef] [PubMed]
- Hagan, D.J.; Chan, D.; Schwalbe, C.H.; Stevens, M.F.G. J. Chem. Soc. Perkin Trans. 1 1998, 915–924. [CrossRef]
- Wamhoff, H. Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: New York, 1984; Vol. 5, p. 669. [Google Scholar]
- Sha, C.-K.; Mohanakrishnan, A.K. The Chemistry of Heterocyclic Compounds; Padwa, A., Pearson, W.H., Eds.; John Wiely: New York, 2002; Vol. 59. [Google Scholar]
- Gilchrist, T.L.; Gymer, G.E. Advances in Hererocyclic Chemistry; Katritzky, A.R., Boulton, A.J., Eds.; Academic Press: New York, 1974; Vol. 16. [Google Scholar]
- Padwa, A. 1,3-Dipolar Cycloaddition Chemistry; Taylor, E.C., Weissberger, A., Eds.; Wiely- Interscience: New York, 1984; Vol. 1. [Google Scholar]
- Sheradsky, T. The Chemistry of the Azido Group; Patai, S., Ed.; Intersicence: New York, 1971; Chap. 6; pp. 882–893. [Google Scholar]
- Savin, K.A.; Robertson, M.; Gernet, D.; Green, S.; Hembre, E.J.; Bishop, J. Mol. Divers. 2003, 7, 171–174. [CrossRef]
- Garanti, L.; Molteni, G. Tetrahedron Lett. 2003, 44, 1133–1135.
- Molteni, G.; Buttero, P.D. Tetrahedron 2005, 61, 4983–4987.
- Alvarez, R.; Velazquez, S.; San, F.; Aquaro, S.; De, C.; Perno, C.; Karlsson, A.; Balzarini, J.; Camarasa, J.M. J. Med. Chem. 1994, 37, 4285.
- Velazquez, S.; Alvarez, R.; Perez, C.; Gago, F.; De, C.; Balzarini, J.; Camarasa, M.J. Antivir. Chem. Chemoter. 1998, 9, 481.
- Genin, M.J.; Allwine, D.A.; Andersn, D.J.; Barbachyn, M.R.; Grega, K.C.; Hester, J.B.; Hutchinson, D.K.; Morris, J.; Reischer, R.J.; Ford, C.W.; Zurenko, G.E.; Hamel, J.C.; Schaadt, R.D.; Stapert, D.; Yagi, B.H. J. Med. Chem. 2000, 43, 953. [CrossRef]
- Appukkuttan, P.; Dehaen, W.; Fokin, V.V.; Van der Eyken, E. Org. Lett. 2004, 6, 4223–4225.
- Guezguez, R.; Bougrin, K.; El Akriand, K.; Benhida, R. Tetrahedron Lett. 2006, 47, 4807–4811.
- Rostotsev, V.V.; Green, L.G.; Flokin, V.V.; Sharpless, K.B. Angew. Chem. Int. Ed. 2002, 41, 2596.
- Tornøe, C.W.; Christensen, C.; Medal, M. J. Org. Chem. 2002, 67, 3057. [CrossRef]
- Chan, T.R.; Hilgraf, R.; Sharpless, K.B.; Fokin, V.V. Org. Lett. 2004, 6, 2853.
- Fu, X.; Albermann, C.; Zhang, C.; Thorson, J.S. Org. Lett. 2005, 7, 1513.
- Bock, V.D.; Hiemstra, H.; van Maarseveen, J.H. Eur. JOC 2006, 1, 51–68.
- Harju, K.; Vahermo, M.; Mutikainen, I.; Yli-Kauhaluoma, J. J. Comb. Chem. 2003, 5, 826–833. [CrossRef] [PubMed]
- Molteni, G.; Del Buttero, P. Tetrahedron 2005, 61, 4983–4987.
- Sternfeld, F.; Carling, R.W.; Jelley, R.A.; Ladduwahetty, T.; Merchant, K.J.; Moore, K.W.; Reeve, A.J.; Street, L.J.; O’Connor, D.; Sohal, B.; Atack, J.R.; Cook, S.; Seabrook, G.; Wafford, K.; Tattersall, F.D.; Collinson, N.; Dawson, G.R.; Castro, J.L.; Macleod, A.M. J. Med. Chem. 2004, 47, 2176–2179. [CrossRef] [PubMed]
- Yasuda, G.; Kimoto, H. J. Heterocyclic Chem. 1998, 35, 365. [CrossRef]
- Barton, J.W.; Grinham, A.R. J. Chem. Soc. Perkin Trans. 1 1972, 634.
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
