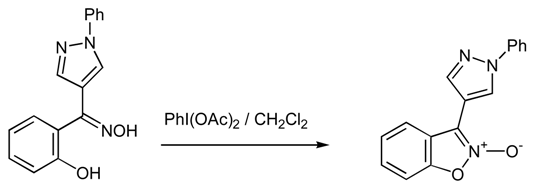
It is well known that both isoxazole ring as well as pyrazole ring possess various biological activities and they both show interesting antimicrobial activity [1,2,3]. Therefore, it is not unreasonable to believe that a molecule bearing both moieties would possible show combined activity. Having this in mind we designed the synthesis of 3-(1-phenyl-1H-pyrazol-4-yl)-1,2-benzisoxazole 2-oxide as shown below. Nitrogen derivatives of o-hydroxyaryl ketones have been proved valuable starting materials in organic synthesis[4]. Thus, we synthesised (2-hydroxyphenyl)(1-phenyl-1H-pyrazol-4-yl)methanone oxime and we subsequently oxidized it with diacetoxy iodobenzene (DIB). The reaction led to the formation of the oxidative cyclisation product, giving the desired 3-(1-phenyl-1H-pyrazol-4-yl)-1,2-benzisoxazole 2-oxide, in 61% yield.
 (2-Hydroxyphenyl)(1-phenyl-1H-pyrazol-4-yl)methanone oxime was prepared according to the literature methods by refluxing (2-hydroxyphenyl)(1-phenyl-1H-pyrazol-4-yl)methanone and hydrochloric hydroxylamine in ethanol in the presence of pyridine whereas commercially available diacetoxy iodobenzene were supplied by Aldrich.
(2-Hydroxyphenyl)(1-phenyl-1H-pyrazol-4-yl)methanone oxime was prepared according to the literature methods by refluxing (2-hydroxyphenyl)(1-phenyl-1H-pyrazol-4-yl)methanone and hydrochloric hydroxylamine in ethanol in the presence of pyridine whereas commercially available diacetoxy iodobenzene were supplied by Aldrich.

Oxidation of (2-hydroxyphenyl)(1-phenyl-1H-pyrazol-4-yl)methanone oxime
0.58 g (1.79 mmol) of DIB are added to a suspension of 0.5 g (1.79 mmol) of (2-hydroxyphenyl)(1-phenyl-1H-pyrazol-4-yl)methanone oxime in 20 ml CH2Cl2 in an ice-bath. The mixture was then magnetically stirred for 3 hrs. Evaporation of the solvent gave an oil which was then subjected to column chromatography (silica gel 70-230 mesh). Elution with a mixture of petroleum ether / ethylacetate 3:1 afforded the desired 3-(1-phenyl-1H-pyrazol-4-yl)-1,2-benzisoxazole 2-oxide as white crystals (0.30 g, 61 %). The product was identified by its 1H NMR, 13C NMR and MS and elemental analysis.
M.p. 179-180 °C.
1H NMR (400 MHz, DMSO-d6): 7.44-7.52 (m, 3H), 7.58-7.70 (m, 3H), 8.04-8.07(m, 2H), 8.32-8.34(d, J=8.0Hz, 1H), 8.67(s, 1H), 9.36(s, 1H).
13C NMR (100 MHz, DMSO-d6): 107.7, 109.5, 118.9, 119.9, 120.1, 121.6, 125.1, 127.3, 127.7, 128.1, 129.6, 130.2, 139.5, 139.7, 149.1, 150.5.
MS m/z (ES+): 300 [M+Na]+, 277 [M]+, 261, 247.
Anal. Calc. for C16H11N3O2: C 69.31, H 4.00, N 15.15; found: C 69.22, H 3.88, N, 15.10
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Priya, B.S.; Bassappa, S.; Nanjunda, S.; Rangappa, S. Biorganic and Med. Chem. 2005, 13, 2623. [CrossRef]
- Menozzi, G.; Merello, L.; Fossa, P.; Schenome, S.; Ranise, A.; Mosti, L.; Bondavalli, F.; Loddo, R.; Murgioni, C.; Mascia, V.; La Colla, P.; Tamburini, E. Bioorg. Med. Chem. 2004, 12(20), 5465. [CrossRef] [PubMed]
- Bekhit, A.A.; Ashour, H.M.; Guemei, A.A. Arch Pharm (Weinheim) 2005, 338(4), 167. [PubMed]
- Kotali, A.; Harris, P.A. Org. Prep. Proc. Int. 1994, 26(2), 155.
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).