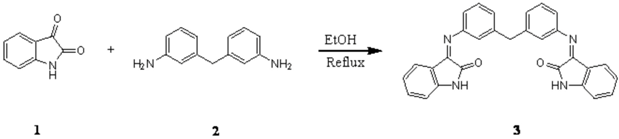
Synthesis of 3,3´-[methylenebis(3,1-phenylenenitrilo)]bis[1,3-dihydro]-2H-indol-2-one as a novel bis-Schiff base
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Dasilva, J. F. M.; Garden, S. J.; Pinto, A. C. J. Braz. Chem. Soc. 2001, 12(3), 273.
- Guo, Y.; Chen, F. Zhongcaoyao 1986, 17, 8, (CA 104:213068f).
- Yoshikawa, M.; Murakami, T.; Kishi, A.; Sakurama, T.; Matsuda, H.; Nomura, M.; Matsuda, H.; Kubo, M. Chem. Pharm. Bull. 1998, 46, 886. [PubMed]
- Gil-Turners, M. S.; Hay, M. E.; Fenical, W. Science 1989, 116.
- Pandeya, S. N.; Yogeeswari, P.; Sriram, D.; De Clercq, E.; Pannecouque, C.; Witvrouw, M. Chemotherapy 1999, 45, 192–196. [PubMed]
- Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. Eur. J. Med. Chem. 2000, 35, 249–255. [PubMed]
- Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. Arzneimittel Forschun./Drug Res. 2000, 50, 55–59.
- Sridhar, S. K.; Pandeya, S. N.; Stables, J. P.; Armes, S. K. Eur. J. Pharm. Sci. 2002, 16, 129. [PubMed]
- Pandeya, S. N.; Sriram, D. Acta Pharm. Turc. 1998, 40, 33.
- Sarangapani, M.; Reddy, V. M. Indian J. Pharm. Sci. 1994, 56, 174.
- Varma, R. S.; Nobles, W. L. J. J. Pharm. Sci. 1975, 64, 881. [PubMed]
- Imam, S. A.; Varma, R. S. Experientia 1975, 31, 1287. [PubMed]
- Varma, R. S.; Khan, I. A. Polish J. Pharmacol. Pharm. 1977, 29, 549.
- Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. Indian J. Pharm. Sci. 1999, 61, 358.
- Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. Sci.Pharm. 1999, 67, 103.
- Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. Pharm. Acta Helv. 1999, 74, 11. [PubMed]
- Varma, R. S.; Nobles, W. L. J. Med. Chem. 1967, 10, 972. [PubMed]
- Singh, S. P.; Shukla, S. K.; Awasthi, L. P. Curr. Sci. 1983, 52, 766.
- Logan, J. C.; Fox, M. P.; Morgan, J. M.; Makohon, A. M.; Pfau, C. J. J. Gen. Virol. 1975, 28, 271. [PubMed]
- Sarciron, S. E.; Audin, P.; Delebre, I.; Gabrion, C.; Petavy, A. F.; Paris, J. J. Pharm. Sci. 1993, 82, 605. [PubMed]
- Et-savi, E. A.; Mostafa, T. B.; Mostafa, B. B. J. Egypt. Soc. Parasitol. 1998, 28, 481.
- Cerchiaro, G.; Micke, G. A.; Tavares, M. F. M.; Ferreira, A. M. D. C. J. Mol. Catal. A: Chem 2004, 221, 29.
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.
Share and Cite
Jarrahpour, A.A.; Khalili, D. Synthesis of 3,3´-[methylenebis(3,1-phenylenenitrilo)]bis[1,3-dihydro]-2H-indol-2-one as a novel bis-Schiff base. Molbank 2005, 2005, M437. https://doi.org/10.3390/M437
Jarrahpour AA, Khalili D. Synthesis of 3,3´-[methylenebis(3,1-phenylenenitrilo)]bis[1,3-dihydro]-2H-indol-2-one as a novel bis-Schiff base. Molbank. 2005; 2005(4):M437. https://doi.org/10.3390/M437
Chicago/Turabian StyleJarrahpour, A. A., and D. Khalili. 2005. "Synthesis of 3,3´-[methylenebis(3,1-phenylenenitrilo)]bis[1,3-dihydro]-2H-indol-2-one as a novel bis-Schiff base" Molbank 2005, no. 4: M437. https://doi.org/10.3390/M437
APA StyleJarrahpour, A. A., & Khalili, D. (2005). Synthesis of 3,3´-[methylenebis(3,1-phenylenenitrilo)]bis[1,3-dihydro]-2H-indol-2-one as a novel bis-Schiff base. Molbank, 2005(4), M437. https://doi.org/10.3390/M437



