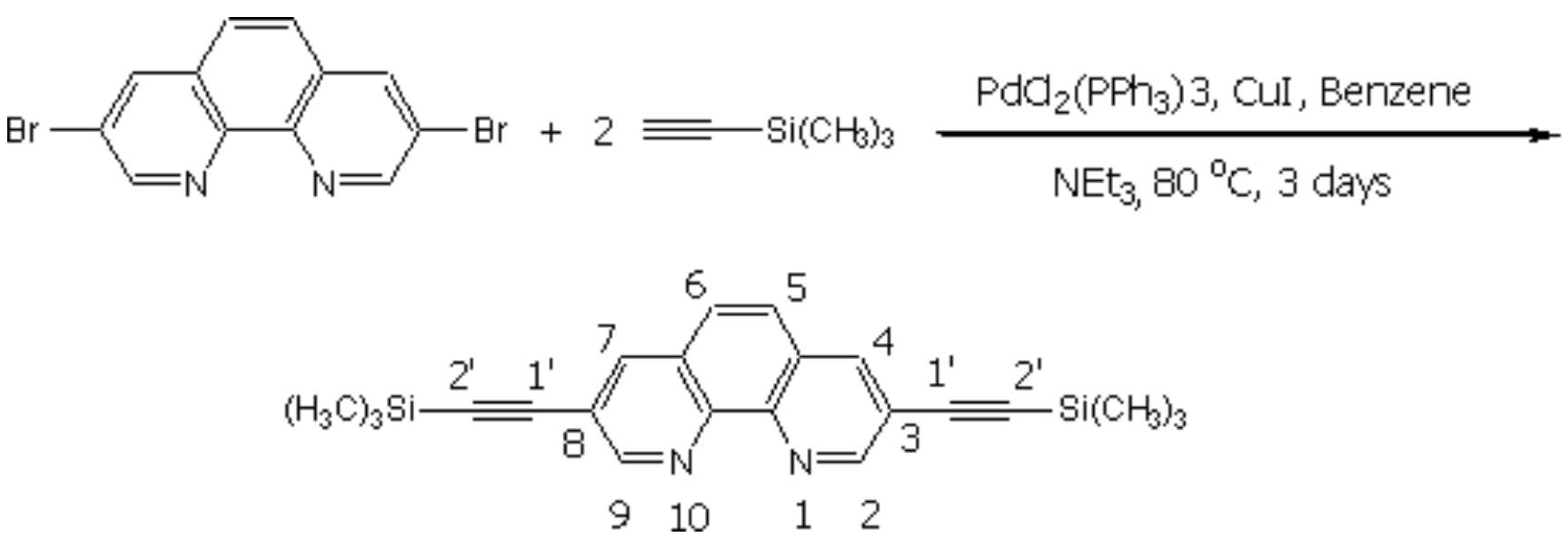
3,8-Bis-trimethylsilanylethynyl-[1,10]-phenanthroline
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Sonogashira, K.; Tohda, Y.; Hagihara, N. Tetrahedron Lett. 1975, 4467.
- Schmittel, M.; Ammon, H. Synlett. 1999, 750.
- Tzalis, D.; Tor, Y.; Failla, S.; Siegel, J.S. Tetrahedron Lett. 1995, 36, 3489.
© 2005 MDPI. All rights reserved.
Share and Cite
Habibi, D. 3,8-Bis-trimethylsilanylethynyl-[1,10]-phenanthroline. Molbank 2005, 2005, M423. https://doi.org/10.3390/M423
Habibi D. 3,8-Bis-trimethylsilanylethynyl-[1,10]-phenanthroline. Molbank. 2005; 2005(3):M423. https://doi.org/10.3390/M423
Chicago/Turabian StyleHabibi, Davood. 2005. "3,8-Bis-trimethylsilanylethynyl-[1,10]-phenanthroline" Molbank 2005, no. 3: M423. https://doi.org/10.3390/M423
APA StyleHabibi, D. (2005). 3,8-Bis-trimethylsilanylethynyl-[1,10]-phenanthroline. Molbank, 2005(3), M423. https://doi.org/10.3390/M423



