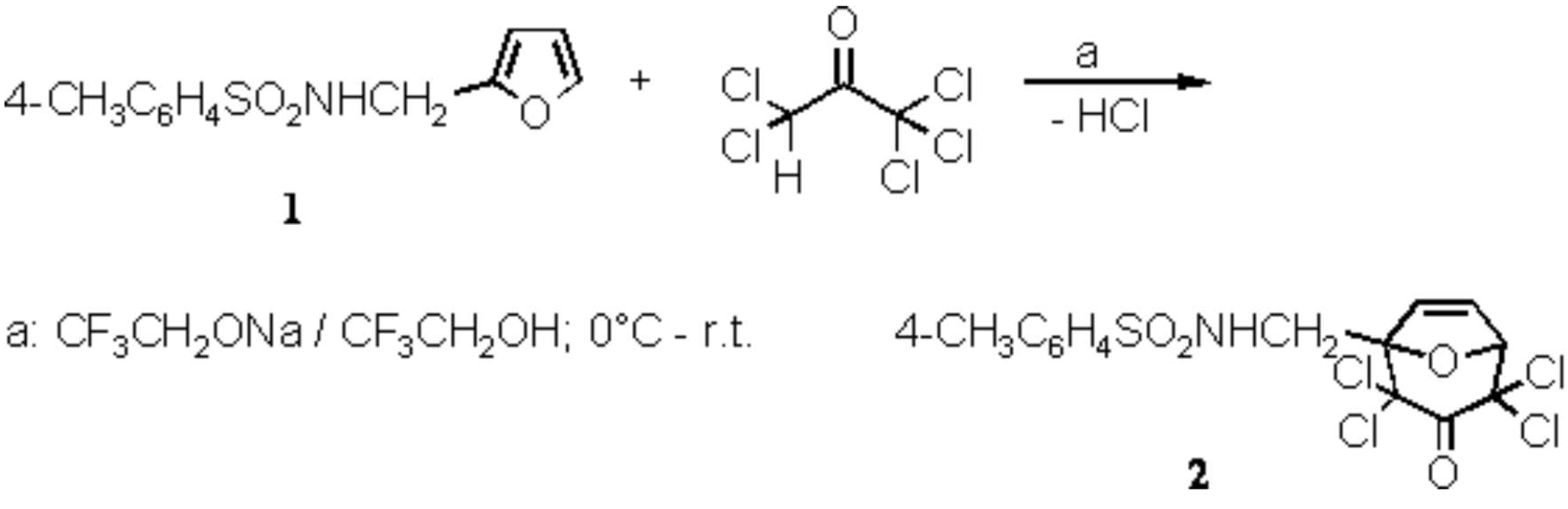
4-Methyl-N-(2,2,4,4-tetrachloro-3-oxo-8-oxabicyclo[3.2.1.]oct-6-en-1-ylmethyl)-benzenesulfonamide
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- a) Chooney, N.; Kuhnert, N.; Sammes, P. G.; Smith, G.; Ward, R. W. J. Chem. Soc., Perkin Trans 1 2002, 1999–2005. b) Meining, H.; Föhlisch, B. Molbank 2005, M407.
- Bugrova, L. V.; Rudnev, G. K.; Kristich, A. I.; Radchenko, V. I.; Mishchenko, L. F. Zh. Prikl. Khim. (Leningrad) 1973, 46, 1529–1533, J. Appl. Chem. USSR 1973, 2, 1627–1631.
- a) Föhlisch, B.; Gehrlach, E.; Herter, R. Angew. Chem. 1982, 94, 144, Angew. Chem. Suppl. 1982, 94, 241; Angew. Chem., Int. Ed. Engl. 1982, 21, 137. b) Sendelbach, S.; Schwetzler-Raschke, R.; Radl, A.; Kaiser, R.; Henle, G. H.; Korfant, H.; Reiner, S.; Föhlisch, B. J. Org. Chem. 1999, 64, 3398–3408. [CrossRef]
- If 1 has not disappeared after that time (check by TLC), more pentachloroacetone (0.7–1.2 g, 3–5 mmol) was added, and the base solution in such amount that a test with wet pH indicator paper showed an alkaline reaction.
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.
Share and Cite
Meining, H.; Föhlisch, B. 4-Methyl-N-(2,2,4,4-tetrachloro-3-oxo-8-oxabicyclo[3.2.1.]oct-6-en-1-ylmethyl)-benzenesulfonamide. Molbank 2005, 2005, M408. https://doi.org/10.3390/M408
Meining H, Föhlisch B. 4-Methyl-N-(2,2,4,4-tetrachloro-3-oxo-8-oxabicyclo[3.2.1.]oct-6-en-1-ylmethyl)-benzenesulfonamide. Molbank. 2005; 2005(2):M408. https://doi.org/10.3390/M408
Chicago/Turabian StyleMeining, Holger, and Baldur Föhlisch. 2005. "4-Methyl-N-(2,2,4,4-tetrachloro-3-oxo-8-oxabicyclo[3.2.1.]oct-6-en-1-ylmethyl)-benzenesulfonamide" Molbank 2005, no. 2: M408. https://doi.org/10.3390/M408
APA StyleMeining, H., & Föhlisch, B. (2005). 4-Methyl-N-(2,2,4,4-tetrachloro-3-oxo-8-oxabicyclo[3.2.1.]oct-6-en-1-ylmethyl)-benzenesulfonamide. Molbank, 2005(2), M408. https://doi.org/10.3390/M408



