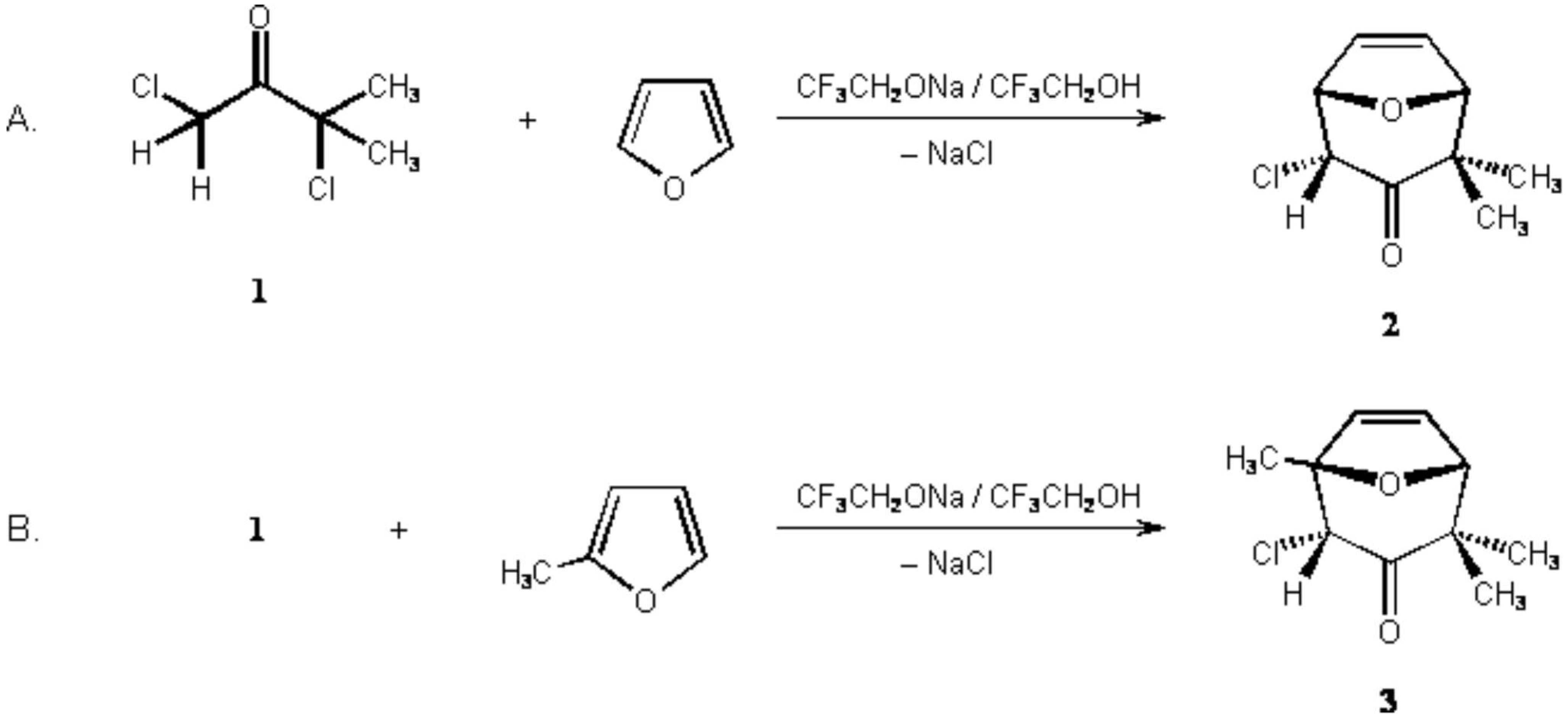
We report the synthesis of (2endo)-2-Chloro-4,4-dimethyl-8-oxabicyclo[3.2.1]oct-6-en-3-one (2) and (2endo)-2-chloro-1,4,4-trimethyl-8-oxabicyclo[3.2.1]oct-6-en-3-one (3) via [4+3] cycloaddition of an oxyallyl intermediate generated from 1,3-dichloro-3-methylbutan-2-one in 2,2,2-trifluoroethanol. Compound 3 was described in the Molbank paper M405.
1. Procedures
A. (2endo)-2-Chloro-4,4-dimethyl-8-oxabicyclo[3.2.1]oct-6-en-3-one (2).
A 250-mL round-bottomed flask, equipped with a magnetic stirring bar and a dropping funnel, is charged with 1,3-dichloro-3-methylbutan-2-one (1, 15.5 g, 100 mmol) (Note 1) and furan (21 mL, 300 mmol) (Note 2). The mixture is chilled in an ice bath. With magnetic stirring, a solution of sodium 2,2,2-trifluoroethoxide (NaTFE) in 2,2,2-trifluoroethanol (TFE) (c = 1 mol/L, 120 mL) (Note 3) is added dropwise within 2 hours. A white precipitate of sodium chloride is formed. The cooling bath is removed, and the mixture is stirred for 2 hr further at room temperature. The sodium chloride is dissolved by addition of water (250 mL), the mixture transferred into a separatory funnel and diluted with diethyl ether (200 mL). The layers are separated, and the inorganic layer is extracted further with ether (3 x 100 mL). The combined organic layers are washed with brine (150 mL), dried over anhydrous sodium sulfate and filtered.The solvent is removed under reduced pressure to give a yellowish solid that is dried in vacuo. The crude product (2, 15.81 g, ca. 84% yield) is sublimed at 70°C/ 0.02 Torr to afford 13.85 g (74%) of (2endo)-2-chloro-4,4-dimethyl-8-oxabicyclo [3.2.1]oct-6-en-3-one as colorless crystals with mp 80–81 °C. This product is pure enough (1H-NMR) for further transformations (Notes 4 and 5).
B. (2endo)-2-Chloro-1,4,4-trimethyl-8-oxabicyclo[3.2.1]oct-6-en-3-one (3).
A 250-mL round-bottomed flask, equipped with a magnetic stirring bar and a dropping funnel, is charged with 1,3-dichloro-3-methylbutan-2-one (1, 7.75 g, 50 mmol) (Note 1) and 2-methylfurane (18 mL, 200 mmol) (Note 6). The mixture is chilled in an ice bath. With magnetic stirring, a solution of sodium 2,2,2-trifluoroethoxide (NaTFE) in 2,2,2-trifluoroethanol (TFE) (c = 1 mol/L, 55 mL) (Note 3) is added dropwise within 2 hours. A white precipitate of sodium chloride is formed. The cooling bath is removed, and the mixture is stirred for 2 hr further at room temperature. The sodium chloride is dissolved by addition of water (200 mL), the mixture transferred into a separatory funnel and diluted with diethyl ether (150 mL). The layers are separated and the inorganic layer is extracted further with ether (3 x 100 mL). The combined organic layers are washed with brine (150 mL), dried over anhydrous sodium sulfate and filtered. The solvent is removed under reduced pressure to give a yellowish solid that is dried in vacuo. The crude product, (9.12 g, ca. 90% yield), is recrystallized from n-heptane (10 mL) to give 6.34 g (63%) of (2endo)-2-chloro-1,4,4-trimethyl-8-oxabicyclo[3.2.1]oct-6-en-3-one (3) as colorless crystals with mp 101–102 °C (Note 7).
2. Notes
- 1,3-Dichloro-3-methylbutan-2-one (1) may be prepared by vapor phase chlorination of 3-methylbutan-2-one (methyl isopropyl ketone).1 Since this preparation requires a special equipment, the authors prepared dichloroketone 1 by reaction of 3-methylbutan-2-one with sulfuryl chloride (SO2Cl2), as described in the preceeding experimental procedure.2
- Furan was purchased from Fluka, Neu-Ulm, Germany. In order to remove phenolic stabilizers it is shaken with 5 percent aqueous potassium hydroxide solution until the aqueous layer remains colorless, dried over calcium chloride and distilled before use from potassium hydroxide pellets.
- 2,2,2-Trifluoroethanol (TFE) is commercially available in high purity (GC >99%, Fluka, puriss., or ABCR, Karlsruhe, Germany) and is used directly, without further purification. A solution of sodium 2,2,2-trifluoroethoxide (NaTFE) is prepared by adding small (!) cut pieces of sodium to TFE at room temperature. Caution: If the sodium is added too rapidly, i.e. the local amount of sodium is too large, overheating can occur; in one of some dozens of preparations, the reaction mixture decomposed with charring and ignition, even under inert gas!In this procedure, a 1 molar NaTFE solution is used. In other [4+3] cycloaddition reactions the concentration was 2 mol/L, in order to minimize the expensive TFE. However, 2 molar solutions are more viscous and some workers feeled that handling in the dropping funnel would be more difficult. Solutions of higher concentration are no more homogeneous and, therefore, are less convenient to add.
- Recrystallization from n-heptane (ca. 2.5 mL / g) gave a product with mp 84–85°C (Ref.3: 85–86°C, Ref.4: 89–90°C). It shows the following spectral characteristics: 1H NMR (500 MHz, CDCl3) d: 1.05 (s, 3 H, endo-4-CH3), 1.38 (s, 3 H, exo-4-CH3), 4.49 (d, 3J1.6 = 1.7 Hz, 1 H, 5-H), 4.76 (d, 3J1,2 = 4.7 Hz, 1 H, 2-H), 5.05 (dd, 3J1,2 = 4.7 Hz, 3J1,7 = 1.7 Hz, 1 H, 1-H), AB sub-spectrum centered at d = 6.45, with dB = 6.42, dA = 6.47, JAB = 6.1 Hz; the lines are split into doublets with 3J1.7 = 3J5.6 = 1.7 Hz (6-H and 7-H); 13C NMR (125.76 MHz, CDCl3) d: 20.2, 24.4, 54.6, 62.3, 82.3, 87.0, 131.9, 135.6, 203.0.
- The mother-liquor from the crystallization was concentrated and subjected to chromatography on a glass column (length 25 cm, diameter 2 cm) packed with 20 g of silica (Macherey & Nagel, Düren, Germany, Kieselgel 60, particle size 63–200 mm). Elution was made with petroleum ether/ethyl acetate (8 : 1). The first fractions, after a fore-run (30 mL), contained 22 mg (1%) of 2-chloro-3-furyl-3-methylbutan-2-one (4), a colorless oil. From the following fractions, 81 mg (2%) of 2, and at last 36 mg of a colorless viscous oil was isolated. According to the NMR, the latter substance was a mixture of several bicyclic compounds, whose structure could not be rigorously identified.
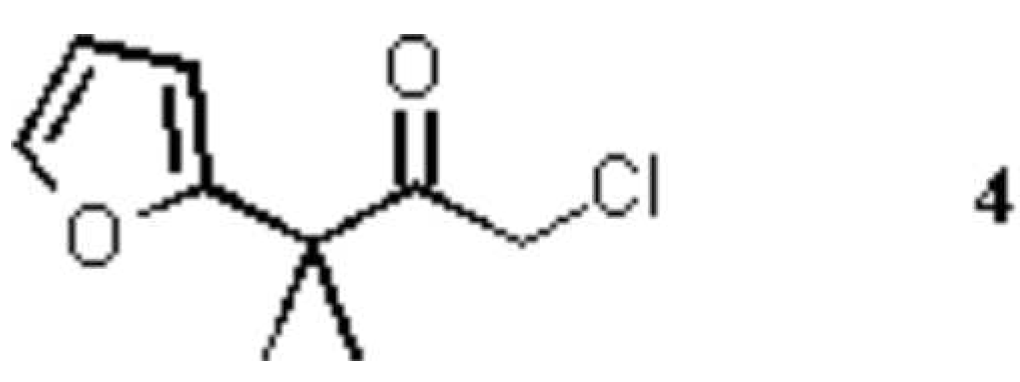 The substituted furan 4 is a known compound4, that was identified by the following spectrum: 1H NMR (250 MHz, CDCl3) d: 1.52 (s, 6 H, 4-H and 3-CH3), 4.10 (s, 2 H, 1-H), ABX sub-spectrum with dB = 6.24 (Furan-3-H), dA = 6.38 (Furan-4-H), dX = 7.40 (Furan-5-H), JAB = 3.3 Hz, JAX = 1.8 Hz, JBX = 0.6 Hz; 13C NMR (62.90 MHz, CDCl3) d: 23.6 (C-4, 3-CH3), 46.3 (C-1), 48.3 (C-3), 106.6, 110.8 (Furan-C-3 and -C-4), 142.6 (Furan-C-5), 156.2 (Furan-C-2), 202.1 (C-2).
The substituted furan 4 is a known compound4, that was identified by the following spectrum: 1H NMR (250 MHz, CDCl3) d: 1.52 (s, 6 H, 4-H and 3-CH3), 4.10 (s, 2 H, 1-H), ABX sub-spectrum with dB = 6.24 (Furan-3-H), dA = 6.38 (Furan-4-H), dX = 7.40 (Furan-5-H), JAB = 3.3 Hz, JAX = 1.8 Hz, JBX = 0.6 Hz; 13C NMR (62.90 MHz, CDCl3) d: 23.6 (C-4, 3-CH3), 46.3 (C-1), 48.3 (C-3), 106.6, 110.8 (Furan-C-3 and -C-4), 142.6 (Furan-C-5), 156.2 (Furan-C-2), 202.1 (C-2). - 2-Methylfuran was purchased from Fluka and treated as described for furan in Note 2.
- The crystalline solid obtained after recrystallization is sufficiently pure for further transformations, according to a 1H NMR spectrum. The product may be purified further by sublimation at 80 °C/ 0.02 Torr, to give a solid with 99% purity (GLC) and mp 103–104°C (Ref.4: 104–105°C).
Waste disposal information
Recycling of TFE from the waste of cycloaddition reactions. The liquid waste resulting from concentration of the diethyl ether phases by distillation in the rotary evaporator is collected. It may turn yellow to brown on storing. Combined waste containing TFE is treated as described in Ref.5 and worked-up to give recycled TFE.
3. Discussion
The preceeding experimental procedure2 of a [4 + 3] cycloaddition reaction illustrates the use of lithium perchlorate/diethylether/triethylamine as a solvent system for the generation of oxyallyl intermediates. In many cases, better yields of cycloadducts have been obtained using 2,2,2-trifluoroethanol (TFE) instead of lithium perchlorate/diethyl ether.5,6,7,8 Triethylamine may be used as the base inducing the generation of oxyallyl intermediates.6,9
The authors prefer sodium trifluoroethoxide (NaTFE), because progress of the reaction can be monitored visually by the precipitation of sodium chloride. Moreover, recycling of the TFE is easier in the absence of triethylamine; the latter forms hydrogen-bridged complexes with TFE10 that may complicate work-up.
This procedure demonstrates the generation and [4+3] cycloaddition of a monochloro oxyallyl intermediate derived from an a,a'-dichloroketone in TFE/NaTFE. The benefits of the TFE protocol have been outlined elsewere.9,11 It is especially suitable for the preparation of halogenated bicyclics as demonstrated by 2.
Compound 2 has been prepared previously using triethylamine/ lithium perchlorate / diethyl ether.3 A yield of 53% has been obtained at a 100 mmol scale. Shimizu et al.4 transformed dichloroketone 1 into the trimethylsilylenol ether. The latter was reacted with furan using silver perchlorate and calcium carbonate in nitromethane for generation of the oxyallyl intermediate. An 89% yield of 2 was reported for a 10 mmol scale. The same reaction, with tetrahydrofuran solvent instead of nitromethane, afforded the bicycle 2, the exo-chloro isomer and 1-chloro-3-furyl-3-methylbutan-2-one (4); the latter is the outcome of an electrophilic substitution of furan by the oxyallyl cation derived from 1.
The oxabicyclic a-chloroketone 2 can be reacted with lithium diisopropylamide (LDA). The chlorosubstituted enolate, thus formed in situ, gives (2endo)-2-chloro-2,4,4-trimethyl-8-oxabicyclo[3.2.1]oct-6-en-3-one upon reaction with methyl iodide, i.e. C-alkylation is effected. Reduction of 2 with LiAlH4 gives (2endo,3endo)-2-chloro-4,4-dimethyl-8-oxabicyclo[3.2.1]oct-6-en-3-ol with high endo selectivity; catalytical hydrogenation ultimately affords (2endo,3endo)-2-chloro-4,4-dimethyl-8-oxabicyclo[3.2.1]octan-3-ol.12 As for bicyclic compound 3 see the preceeding preparation.2
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References
- Rabjohn, N.; Rogier, E. R. J. Org. Chem. 1946, 11, 781. [CrossRef]
- Oexle, J.; Buchinger, M.; Föhlisch, B. Molbank 2005, ####.
- Herter, R.; Föhlisch, B. Synthesis 1982, 976.
- Shimizu, N.; Tanaka, M.; Tsuno, Y. J. Am. Chem. Soc. 1982, 104, 1330. [CrossRef]
- Sendelbach, S.; Schwetzler-Raschke, R.; Radl, A.; Kaiser, R.; Henle, G. H.; Korfant, H.; Reiner, S.; Föhlisch, B. J. Org. Chem. 1999, 64, 3398. [CrossRef]
- Föhlisch, B.; Gehrlach, E.; Herter, R. Angew. Chem. 1982, 94, 144, Angew. Chem. Suppl. 1982, 241; Angew. Chem. Internat. Edit. Engl. 1982, 21, 137.
- Föhlisch, B.; Gehrlach, E.; Henle, G. H. J. Chem. Res. (M) 1991, 1462, J. Chem. Res. (S) 1991, 136.
- Föhlisch, B.; Korfant, H.; Meining, H.; Frey, W. Eur. J. Org. Chem. 2000, 1335.
- Jin, S.-J.; Choi, J.-R.; Oh, J.; Lee, D.; Cha, J. K. J. Am. Chem. Soc. 1995, 117, 10914. [CrossRef]
- (a) Zdzienski, H. K.; Wood, J. L. J. Chem. Soc. Faraday Trans. 2 1974, 409. (b) Hussein, M. A.; Millen, D. J. J. Chem. Soc. Faraday Trans. 2 1974, 685.
- Rigby, J. H.; Pigge, F. C. Org. React. 1997, 51, 351.
- Föhlisch, B.; Kreiselmeier, G. Tetrahedron 2001, 57, 10077.
