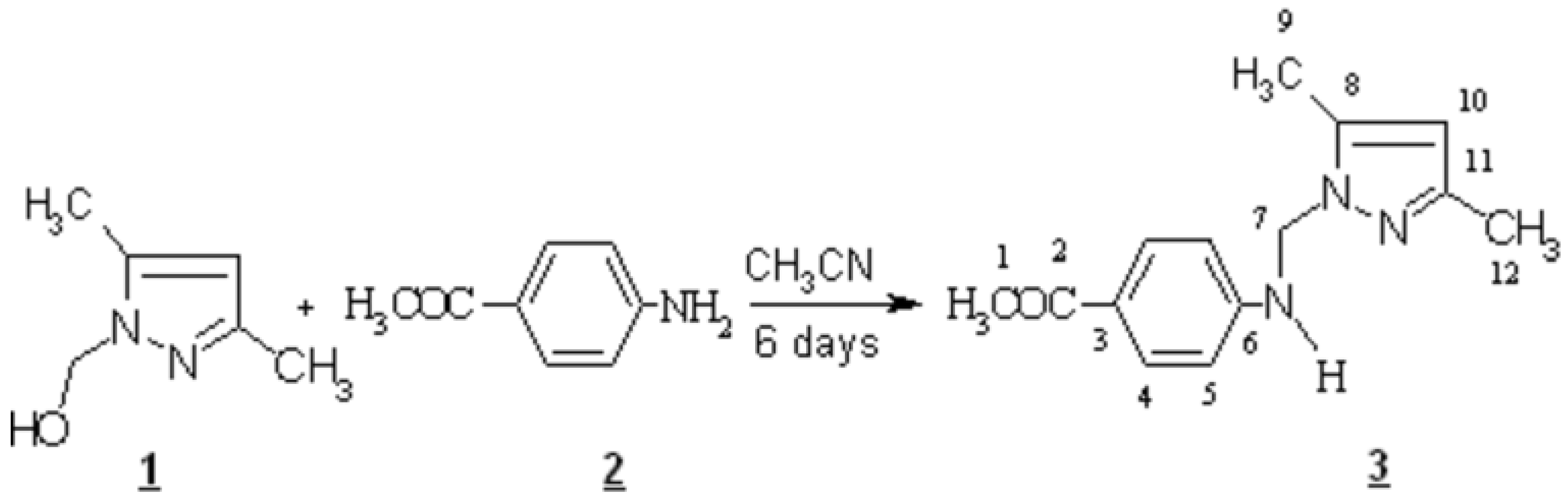
1-(4-{[(3,5-dimethyl-1H-pyrazol-1-yl)methyl] amino} phenyl) ethanone
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Sorrel, T. N.; Vankai, V. A.; Garrity, M. L. Inorg. Chem. 1991, 30, 207.
- Malachowski, M. R.; Davidson, M. G.; Hoffman, J. N. Inorg. Chim. Acta 1989, 157, 91.
- Sheu, S. C.; Tien, M. J.; Cheng, M. C.; Ho, T. I.; Peng, S. M.; Lin, Y. C. J. Chem. Soc. Dalton Trans. 1995, 3503.
- Touzani, R.; Ramdani, A.; Ben-Hadda, T.; El Kadiri, S.; Maury, O.; Le Bozec, H.; Dixneuf, P. H. Synth. Commun. 2001, 31, 1315.
- Sample Availability : Available from the Authors.
© 2004 MDPI. All rights reserved
Share and Cite
El Kodadi, M.; Malek, F.; Ramdani, A. 1-(4-{[(3,5-dimethyl-1H-pyrazol-1-yl)methyl] amino} phenyl) ethanone. Molbank 2004, 2004, M369. https://doi.org/10.3390/M369
El Kodadi M, Malek F, Ramdani A. 1-(4-{[(3,5-dimethyl-1H-pyrazol-1-yl)methyl] amino} phenyl) ethanone. Molbank. 2004; 2004(1):M369. https://doi.org/10.3390/M369
Chicago/Turabian StyleEl Kodadi, Mohamed, Fouad Malek, and Abdelkrim Ramdani. 2004. "1-(4-{[(3,5-dimethyl-1H-pyrazol-1-yl)methyl] amino} phenyl) ethanone" Molbank 2004, no. 1: M369. https://doi.org/10.3390/M369
APA StyleEl Kodadi, M., Malek, F., & Ramdani, A. (2004). 1-(4-{[(3,5-dimethyl-1H-pyrazol-1-yl)methyl] amino} phenyl) ethanone. Molbank, 2004(1), M369. https://doi.org/10.3390/M369



