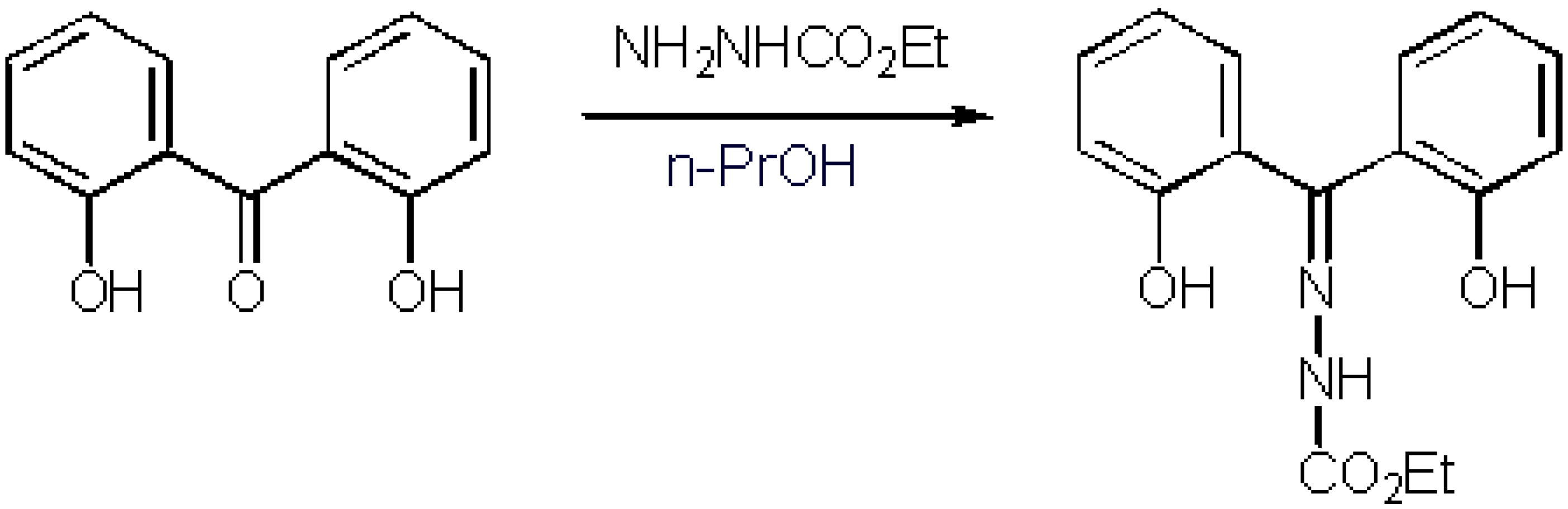
1-(o-Hydroxyphenyl)-3-phenylpropenone N-Benzoylhydrazone
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Kotali, A.; Harris, P. A. Org. Prep. Proc. Int. 1994, 26, 159–192. [CrossRef]
- Kotali, A. Cur. Org. Chem. 2002, 6, 965–981.
- Sample availability: available from the authors and MDPI.
© 2003 MDPI. All rights reserved.
Share and Cite
Kotali, A. 1-(o-Hydroxyphenyl)-3-phenylpropenone N-Benzoylhydrazone. Molbank 2003, 2003, M350. https://doi.org/10.3390/M350
Kotali A. 1-(o-Hydroxyphenyl)-3-phenylpropenone N-Benzoylhydrazone. Molbank. 2003; 2003(4):M350. https://doi.org/10.3390/M350
Chicago/Turabian StyleKotali, Antigoni. 2003. "1-(o-Hydroxyphenyl)-3-phenylpropenone N-Benzoylhydrazone" Molbank 2003, no. 4: M350. https://doi.org/10.3390/M350
APA StyleKotali, A. (2003). 1-(o-Hydroxyphenyl)-3-phenylpropenone N-Benzoylhydrazone. Molbank, 2003(4), M350. https://doi.org/10.3390/M350



