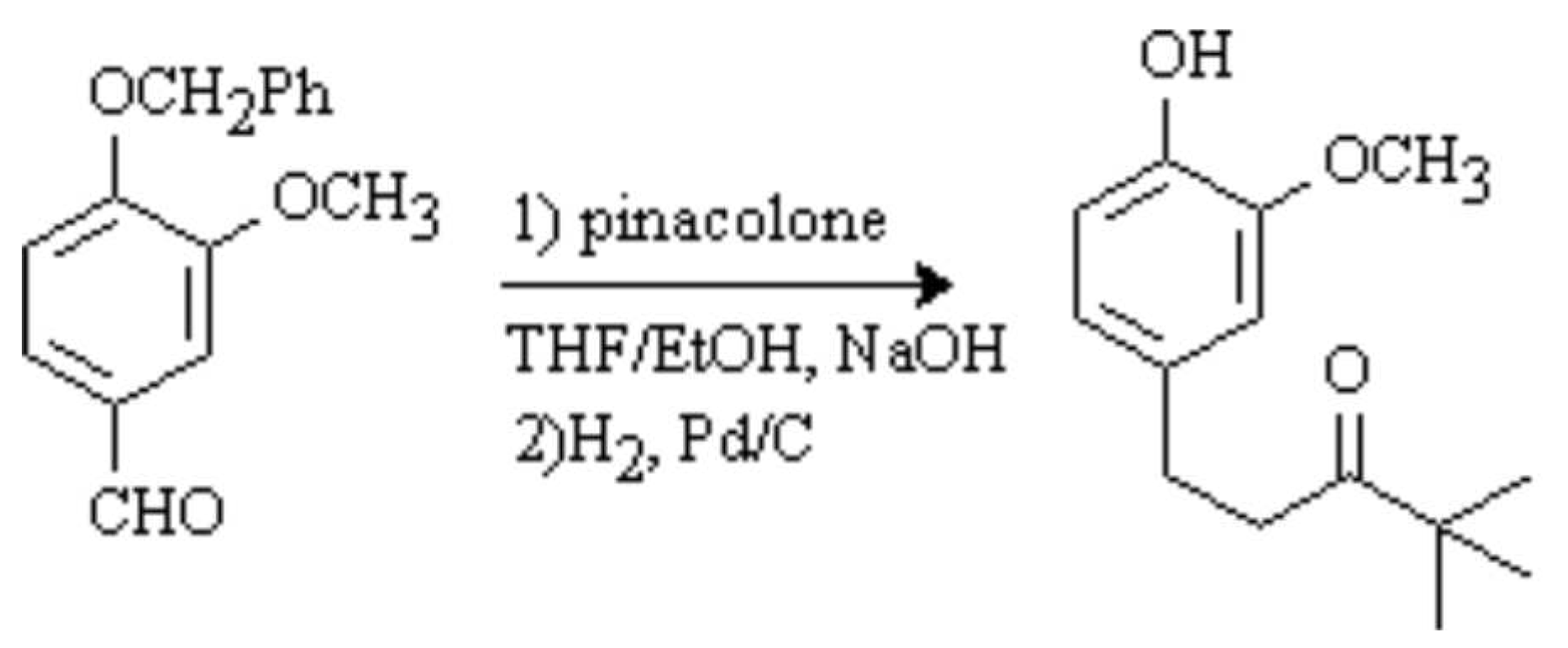
1-(4-Hydroxy-3-methoxyphenyl)-4,4-dimethyl-3-pentanone
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowlegment
References
- Plourde, G.L. Tetrahedron Letters 2002, 43, 3597–3599.
© 2003 MDPI. All rights reserved.
Share and Cite
Plourde, G.L. 1-(4-Hydroxy-3-methoxyphenyl)-4,4-dimethyl-3-pentanone. Molbank 2003, 2003, M320. https://doi.org/10.3390/M320
Plourde GL. 1-(4-Hydroxy-3-methoxyphenyl)-4,4-dimethyl-3-pentanone. Molbank. 2003; 2003(2):M320. https://doi.org/10.3390/M320
Chicago/Turabian StylePlourde, Guy L. 2003. "1-(4-Hydroxy-3-methoxyphenyl)-4,4-dimethyl-3-pentanone" Molbank 2003, no. 2: M320. https://doi.org/10.3390/M320
APA StylePlourde, G. L. (2003). 1-(4-Hydroxy-3-methoxyphenyl)-4,4-dimethyl-3-pentanone. Molbank, 2003(2), M320. https://doi.org/10.3390/M320



