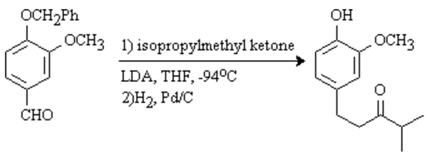
![Molbank 2003 m317 i001]()
The discussion and purpose for the synthesis of this compound has been reported elsewhere [
1]. To a cold (0°C) solution of diisopropylamine (0.72 mL, 520 mg, 5.1 mmol, 1.25 eq) in dry THF (20 mL) was added under an atmosphere of N
2 a solution of BuLi in hexane (1.6 M, 3.2 mL, 5.1 mmol, 1.25 eq) and the solution was stirred at 0°C for 30 min. The solution was cooled to -94°C (acetone/N
2), isopropylmethyl ketone (0.55 mL, 443 mg, 5.1 mmol, 1.25 eq) was added and the solution was stirred at -94°C for 1 h. 4-Benzyloxy-3-methoxybenzaldehyde (985 mg, 4.1 mmol) was added, the suspension was allowed to warm up to 0°C and stirred at that temperature for 3 h. 10% HCl (10 mL) was added, the solution was stirred at room temperature for 1 h and extracted with dichloromethane (3 x 35 mL). The organic fractions were combined, dried (MgSO
4) and the solvent was evaporated in vacuo to give a yellow oil. The crude product was partially purified by chromatography on silica gel (15% EtOAc/hexanes) to give an oil. The oil was dissolved in EtOAc (20 mL), Pd/C (52 mg) was added and the solution was stirred under a positive atmosphere of H
2 for 2 h. The solution was filtered through celite and the solvent was evaporated in vacuo. Chromatography on silica gel (20% EtOAc/hexanes) afforded a clear oil (196 mg, 22%).
IR (neat) cm-1: 3439 (OH), 1709 (CO).
1H-NMR (CDCl3) d: 1.07 (d, 6H, J=6.9 Hz, CH3), 2.54 (m, 1H, H-4), 2.76 (m, 4H, H-1 and H-2), 3.87 (s, 3H OCH3), 5.49 (s, 1H, exchangeable with D2O, OH), 6.67 (m, 2H, ArH-6, ArH-2), 6.81 (d, 1H, J=7.8 Hz, ArH-5).
13C-NMR (CDCl3) d: 18.3 (C-5), 29.8 (C-2), 41.2 (C-4), 42.6 (C-1), 56.1 (OCH3), 111.2 (ArC-2), 114.5 (ArC-5), 121.0 (ArC-6), 133.5 (ArC-1), 144.0 (ArC-4), 146.5 (ArC-3), 214.3 (CO).
MS m/e (rel %): 222 [M+] (50), 179 (34), 151 (17), 137 (100), 119 (11).
Anal. calc. for C13H18O3 C 70.23, H 8.17, found C 70.31, H 8.16.