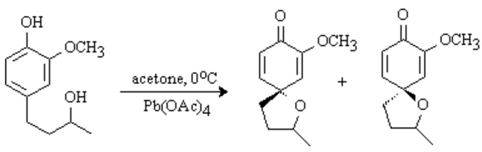
The discussion and purpose for the synthesis of this compound has been reported elsewhere [
1]. To a cold (0°C) solution of (±)-1-(4-hydroxy-3-methoxyphenyl)-3-butanol (106 mg, 0.54 mmol) in acetone (10 mL) was added in one portion Pb(OAc)
4 (655 mg, 1.5 mmol, 2.8 eq). The resulting orange mixture was stirred at 0°C for 2 h. The precipitate was filtered through celite and ethylene glycol (5 drops) was added. The solution was stirred at room temperature for 20 h and filtered through celite. The solvent was evaporated in vacuo to afford a racemic mixture of diastereomers (58/42 ratio). Chromatography on silica gel (20% EtOAc/hexanes) afforded a mixture of diastereoisomers as a colorless oil (65 mg, 62%). Spectroscopic data were obtained from the diastereomeric mixture.
IR (neat) cm-1: 1682 (CO), 1675 (CO).
1H-NMR (CDCl3) d: Major: 1.35 (d, 3H, J=6.1 Hz, CH3), 1.79 (m, 1H, H-3a), 2.17 (m, 3H, H-3b, H-4), 3.68 (s, 3H, OCH3), 4.38 (m, 1H, H-2), 5.75 (d, 1H, J=2.7 Hz, H-6), 6.13 (d, 1H, J=10.0 Hz, H-9), 6.80 (dd, 1H, J=2.7, 10.0 Hz, H-10); Minor: 1.37 (d, 3H, J=6.1 Hz, CH3), 1.79 (m, 1H, H-3a), 2.17 (m, 3H, H-3b, H-4), 3.69 (s, 3H, OCH3), 4.38 (m, 1H, H-2), 5.70 (d, 1H, J=2.7 Hz, H-6), 6.14 (d, 1H, J=10.0 Hz, H-9), 6.86 (dd, 1H, J=2.7, 10.0 Hz, H-10).
13C-nmr (CDCl3) d: Major: 21.6 (CH3), 34.2 (C-3), 38.1 (C-4), 54.9 (OCH3), 76.7 (C-5), 79.6 (C-2), 117.3 (C-6), 125.9 (C-9), 149.8 (C-7), 151.3 (C-10), 181.7 (CO); Minor: 21.5 (CH3), 34.0 (C-3), 37.8 (C-4), 54.9 (OCH3), 76.8 (C-5), 79.6 (C-2), 117.8 (C-6), 125.9 (C-9), 149.8 (C-7), 150.7 (C-10), 181.7 (CO).
MS m/e (rel %): Major: 194 [M+] (100), 179 (34), 166 (29), 151 (61), 139 (33), 123 (33), 111 (44), 85 (73); Minor: 194 [M+] (100), 177 (8), 153 (85), 147 (16), 124 (11).
Anal. calc. for C11H14O3: C 68.01, H 7.27; found: C 67.99 , H 7.52.