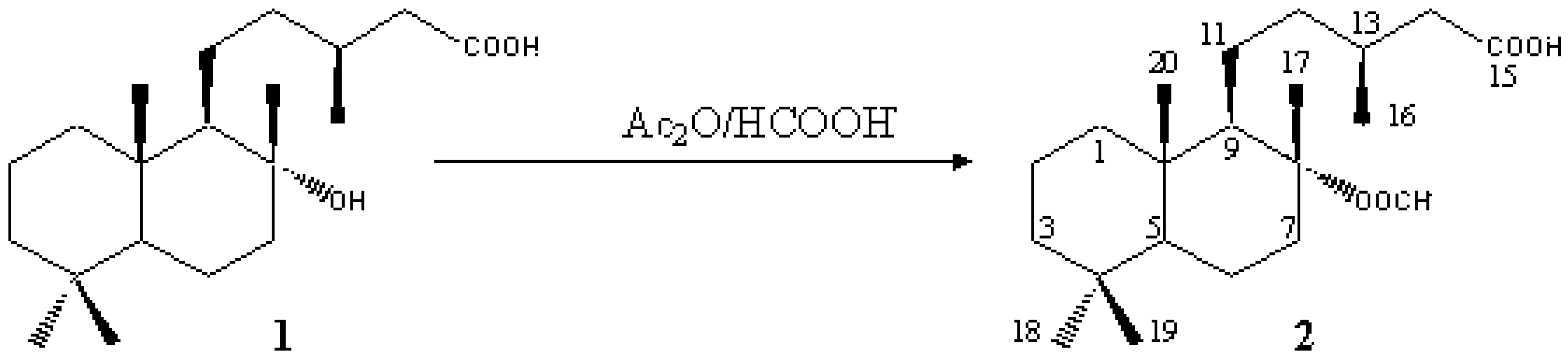
8-Formyl-labdanolic Acid (-)-(3S)-5-((1R,2R,4aS,8aS)-2-Formyloxy-2,5,5,8a-tetramethyldecahydro-1-naphthalenyl)-3-methylpentanoic Acid
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Strazzolini, P.; Giumanini, A. G.; Cauci, S. Acetic Formic Anhydride. A Review. Tetrahedron 1990, 46, 1081–1118. [Google Scholar] [CrossRef]
- Urones, J. G.; Basabe, P.; Marcos, I. S.; González, J. L.; Jiménez, V.; Sexmero, M. J.; Lithgow, A. M. Ambergris Compounds from Labdanolic Acid. Tetrahedron 1992, 48, 9991–9998. [Google Scholar] [CrossRef]
- Sample availability: Available from the authors and from MDPI
© 2003 MDPI. All rights reserved.
Share and Cite
Castro, J.M.; Salido, S.; Altarejos, J.; Nogueras, M.; Sanchez, A. 8-Formyl-labdanolic Acid (-)-(3S)-5-((1R,2R,4aS,8aS)-2-Formyloxy-2,5,5,8a-tetramethyldecahydro-1-naphthalenyl)-3-methylpentanoic Acid. Molbank 2003, 2003, M299. https://doi.org/10.3390/M299
Castro JM, Salido S, Altarejos J, Nogueras M, Sanchez A. 8-Formyl-labdanolic Acid (-)-(3S)-5-((1R,2R,4aS,8aS)-2-Formyloxy-2,5,5,8a-tetramethyldecahydro-1-naphthalenyl)-3-methylpentanoic Acid. Molbank. 2003; 2003(1):M299. https://doi.org/10.3390/M299
Chicago/Turabian StyleCastro, Juan M., Sofia Salido, Joaquin Altarejos, Manuel Nogueras, and Adolfo Sanchez. 2003. "8-Formyl-labdanolic Acid (-)-(3S)-5-((1R,2R,4aS,8aS)-2-Formyloxy-2,5,5,8a-tetramethyldecahydro-1-naphthalenyl)-3-methylpentanoic Acid" Molbank 2003, no. 1: M299. https://doi.org/10.3390/M299
APA StyleCastro, J. M., Salido, S., Altarejos, J., Nogueras, M., & Sanchez, A. (2003). 8-Formyl-labdanolic Acid (-)-(3S)-5-((1R,2R,4aS,8aS)-2-Formyloxy-2,5,5,8a-tetramethyldecahydro-1-naphthalenyl)-3-methylpentanoic Acid. Molbank, 2003(1), M299. https://doi.org/10.3390/M299



