Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review
Abstract
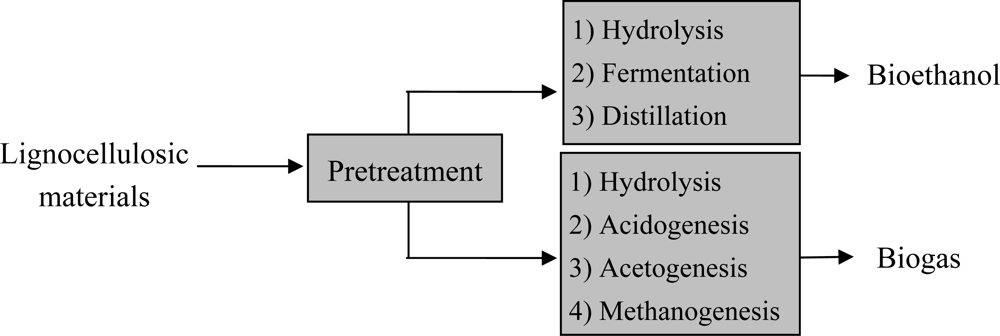
:1. Introduction
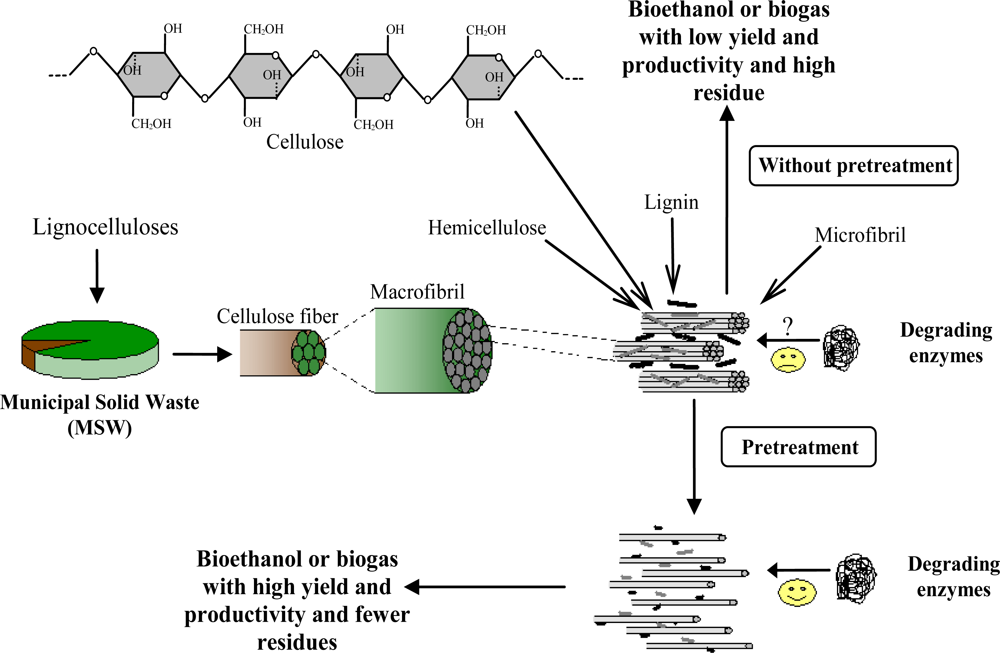
2. Lignocelluloses among waste materials
3. Effective parameters in pretreatment of lignocelluloses
3.1. Crystallinity
3.2. Effect of accessible surface area
3.3. Effect of lignin
3.4. Effect of hemicellulose
4. Pretreatment methods for lignocellulose wastes
4.1. Physical pretreatment
4.1.1. Milling
4.1.2. Irradiation
4.2. Physico-chemical pretreatment
4.2.1. Steam explosion (autohydrolysis)
4.2.2. Steam explosion with addition of SO2
4.2.3. Ammonia fiber explosion (AFEX)
4.2.4. CO2 explosion
4.2.5. Liquid hot-water pretreatment
4.2.6. Microwave-chemical pretreatment
4.3. Chemical pretreatment
4.3.1. Alkaline hydrolysis
4.3.2. Alkaline peroxide
4.3.3. Organosolv process
4.3.4. Wet oxidation
4.3.5. Ozonolysis pretreatment
4.3.6. Acid hydrolysis pretreatment
4.4. Biological pretreatment
5. Concluding remarks
Acknowledgments
References
- Isa, B; Post, J; Furedy, C. Solid Waste Management and Recycling; Actors, Partnerships and Policies in Hyderabad, India and Nairobi, Kenya; Kluwer Academic Publishers: Dordrecht, London, UK, 2004. [Google Scholar]
- Licht, FO. World ethanol markets: the outlook to 2015; Agra Europe special report: Tunbridge Wells, 2006. [Google Scholar]
- Taherzadeh, MJ; Karimi, K. Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 2, 472–499. [Google Scholar]
- Taherzadeh, MJ; Karimi, K. Enzymatic-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 2, 707–738. [Google Scholar]
- Sims, R. Biomass and resources bioenergy options for a cleaner environment in developed and developing countries; Elsevier Science: London, UK, 2003. [Google Scholar]
- Ghosh, S; Henry, MP; Sajjad, A; Mensinger, MC; Arora, JL. Pilot-scale gasification of municipal solid wastes by high-rate and two-phase anaerobic digestion (TPAD). Water Sci. Technol 2000, 41, 101–110. [Google Scholar]
- Buffiere, P; Loisel, D; Bernet, N; Delgenes, JP. Towards new indicators for the prediction of solid waste anaerobic digestion properties. Water Sci. Technol 2006, 53, 233–241. [Google Scholar]
- Sjöström, E. Wood chemistry: fundamentals and applications; Academic Press: San Diego, USA, 1993. [Google Scholar]
- Delmer, DP; Amor, Y. Cellulose biosynthesis. Plant Cell 1995, 7, 987–1000. [Google Scholar]
- Morohoshi, N. Chemical characterization of wood and its components. In Wood and cellulosic chemistry; Hon, DNS, Shiraishi, N, Eds.; Marcel Dekker, Inc: New York, USA, 1991; pp. 331–392. [Google Scholar]
- Ha, MA; Apperley, DC; Evans, BW; Huxham, IM; Jardine, WG; Vietor, RJ; Reis, D; Vian, B; Jarvis, MC. Fine structure in cellulose microfibrils: NMR evidence from onion and quince. Plant J 1998, 16, 183–190. [Google Scholar]
- Talebnia, F; Bafrani, MP; Lundin, M; Taherzadeh, MJ. Optimization study of citrus wastes saccharification by dilute acid hydrolysis. BioResources 2008, 3, 108–122. [Google Scholar]
- Persson, T; Matusiak, M; Zacchi, G; Jonsson, A-S. Extraction of hemicelluloses from process water from the production of masonite. Desalination 2006, 199, 411–412. [Google Scholar]
- Lavarack, BP; Giffin, GJ; Rodman, D. The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose, and other products. Biomass Bioenerg 2002, 23, 367–380. [Google Scholar]
- Emmel, A; Mathias, AL; Wypych, F; Ramos, LP. Fractionation of Eucalyptus grandis chips by dilute acid- catalysed steam explosion. Bioresource Technol 2003, 86, 105–115. [Google Scholar]
- Ademark, P; Varga, A; Medve, J; Harjunpaa, V; Drakenberg, T; Tjerneld, F; Stalbrand, H. Softwood hemicellulose-degrading enzymes from Aspergillus niger: purification and properties of a beta-mannanase. J. Biotechnol 1998, 63, 199–210. [Google Scholar]
- Mod, RR; Ory, RL; Morris, NM; Normand, FL. Chemical properties and interactions of rice hemicellulose with trace minerals in vitro. J. Agr. Food Chem 1981, 29, 449–454. [Google Scholar]
- O’Dwyer, MH. The hemicelluloses of the wood of English oak: The composition and properties of hemicellulose A, isolated from samples of wood dried under various conditions. Biochem. J 1934, 28, 2116–2124. [Google Scholar]
- Taherzadeh, MJ. Ethanol from lignocellulose: physiological effects of inhibitors and fermentation strategies, Ph.D thesis in Biotechnology, Chemical Reaction Engineering, Chalmers University of Technology. 1999.
- Palmqvist, E; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresource Technol 2000, 74, 25–33. [Google Scholar]
- Torget, R; Himmel, ME; Grohmann, K. Dilute sulfuric acid pretreatment of hardwood bark. Bioresource Technol 1991, 35, 239–246. [Google Scholar]
- Donghai, S; Junshe, S; Ping, L; Yanping, L. Effects of different pretreatment modes on the enzymatic digestibility of corn leaf and corn stalk. Chinese J. Chem. Eng 2006, 14, 796–801. [Google Scholar]
- Wyman, CE. Handbook on bioethanol: production and utilization; Taylor & Francis: Washington DC, USA, 1996. [Google Scholar]
- Chum, HL; Douglas, LJ; Feinberg, DA; Schroeder, HA. Evaluation of pretreatments of biomass for enzymatic hydrolysis of cellulose; Solar Energy Research Institute: Golden, Colorado, 1985; pp. 1–64. [Google Scholar]
- Fan, LT; Lee, Y; Beardmore, DH. Mechanism of the enzymatic hydrolysis of cellulose: Effects of major structural features of cellulose on enzymatic hydrolysis. Biotechnol. Bioeng 1980, 22, 177–199. [Google Scholar]
- Grethelin, HE. The effect of pore size distribution on the rate of enzymatic hydrolysis of cellulosic substrates. Biotechnol 1985, 3, 155–160. [Google Scholar]
- Kim, S; Holtzapple, MT. Effect of structural features on enzyme digestibility of corn stover. Bioresource Technol 2006, 97, 583–91. [Google Scholar]
- Sun, Y; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresource Technol 2002, 83, 1–11. [Google Scholar]
- Stone, JE; Scallan, AM; Donefer, E; Ahlgren, E. Cellulases and their Applications; Hajny, GJ, Reese, ET, Eds.; American Chemical Society: Washington DC, USA, 1969. [Google Scholar]
- Berlin, A; Balakshin, M; Gilkes, N; Kadla, J; Maximenko, V; Kubo, S; Saddler, J. Inhibition of cellulase, xylanase and beta-glucosidase activities by softwood lignin preparations. J. Biotechnol 2006, 125, 198–209. [Google Scholar]
- Ramos, LP; Breuil, C; Saddler, JN. Comparison of steam pretreatment of eucalyptus, aspen, and spruce wood chips and their enzymic hydrolysis. Appl. Biochem. Biotechnol. 1992, 37–48. [Google Scholar]
- Mooney, CA; Mansfield, SD; Touhy, MG; Saddler, JN. The effect of initial pore volume and lignin content on the enzymatic hydrolysis of softwoods. Bioresource Technol 1998, 64, 113–119. [Google Scholar]
- Saha, BC; Iten, LB; Cotta, MA; Wu, YV. Dilute acid pretreatment, enzymatic saccharification, and fermentation of rice hulls to ethanol. Biotechnol. Progr 2005, 21, 816–822. [Google Scholar]
- Karimi, K; Kheradmandinia, S; Taherzadeh, MJ. Conversion of rice straw to sugars by dilute-acid hydrolysis. Biomass Bioenerg 2006, 30, 247–253. [Google Scholar]
- Sanchez, G; Pilcher, L; Roslander, C; Modig, T; Galbe, M; Liden, G. Dilute-acid hydrolysis for fermentation of the Bolivian straw material Paja Brava. Bioresource Technol 2004, 93, 249–256. [Google Scholar]
- Schell, DJ; Farmer, J; Newman, M; McMillan, JD. Dilute-sulfuric acid pretreatment of corn stover in pilot-scale reactor: Investigation of yields, kinetics, and enzymatic digestibilities of solids. Appl. Biochem. Biotechnol 2003, 105, 69–85. [Google Scholar]
- Tucker, MP; Kim, KH; Newman, MM; Nguyen, QA. Effects of temperature and moisture on dilute-acid steam explosion pretreatment of corn stover and cellulase enzyme digestibility. Appl. Biochem. Biotechnol 2003, 105, 165–177. [Google Scholar]
- Nguyen, QA; Tucker, MP; Keller, FA; Eddy, FP. Two-stage dilute-acid pretreatment of softwoods. Appl. Biochem. Biotechnol 2000, 84–86, 561–576. [Google Scholar]
- Lee, YY; Iyer, P; Torget, RW. Dilute-acid hydrolysis of lignocellulosic biomass. Adv. Biochem. Eng. Biotechnol 1999, 65, 93–115. [Google Scholar]
- Barl, B; Biliaderis, CG; Murray, ED; Macgregor, AW. Combined chemical and enzymatic treatments of corn husk lignocellulosics. J. Sci. Food Agric 1991, 56, 195–214. [Google Scholar]
- Arato, C; Pye, EK; Gjennestad, G. The lignol approach to biorefining of woody biomass to produce ethanol and chemicals. Appl. Biochem. Biotechnol 2005, 123, 871–882. [Google Scholar]
- Sidiras, D; Koukios, E. Simulation of acid-catalysed organosolv fractionation of wheat straw. Bioresource Technol 2004, 94, 91–98. [Google Scholar]
- Alizadeh, H; Teymouri, F; Gilbert, TI; Dale, BE. Pretreatment of switchgrass by ammonia fiber explosion (AFEX). Appl. Biochem. Biotechnol 2005, 124, 1133–41. [Google Scholar]
- Vlasenko, EY; Ding, H; Labavitch, JM; Shoemaker, SP. Enzymatic hydrolysis of pretreated rice straw. Bioresource Technol 1997, 59, 109–119. [Google Scholar]
- Dale, BE; Leong, CK; Pham, TK; Esquivel, VM; Rios, I; Latimer, VM. Hydrolysis of lignocellulosics at low enzymes level: application of the afex process. Bioresource Technol 1996, 56, 111–116. [Google Scholar]
- Holtzapple, MT; Jun, JH; Ashok, G; Patibandla, SL; Dale, BE. The ammonia freeze explosion (AFEX) process – A practical lignocellulose pretreatment. Appl. Biochem. Biotechnol 1991, 28, 59–74. [Google Scholar]
- Ballesteros, I; Oliva, JM; Navarro, AA; Gonzalez, A; Carrasco, J; Ballesteros, M. Effect of chip size on steam explosion pretreatment of softwood. Appl. Biochem. Biotechnol 2000, 84, 97–110. [Google Scholar]
- Ogier, JC; Ballerini, D; Leygue, JP; Rigal, L; Pourquie, J. Ethanol production from lignocellulosic biomass. Oil Gas Sci. Technol 1999, 54, 67–94. [Google Scholar]
- Boussaid, A; Robinson, J; Cai, YJ; Gregg, DJ; Saddler, JR. Fermentability of the hemicellulose-derived sugars from steam-exploded softwood (Douglas fir). Biotechnol. Bioeng 1999, 64, 284–289. [Google Scholar]
- Sassner, P; Galbe, M; Zacchi, G. Steam pretreatment of Salix with and without SO2 impregnation for production of bioethanol. Appl. Biochem. Biotechnol 2005, 121, 1101–1117. [Google Scholar]
- Ohgren, K; Galbe, M; Zacchi, G. Optimization of steam pretreatment of SO2-impregnated corn stover for fuel ethanol production. Appl. Biochem. Biotechnol 2005, 121, 1055–1067. [Google Scholar]
- Tengborg, C; Stenberg, K; Galbe, M; Zacchi, G; Larsson, S; Palmqvist, E; Hahn-Hägerdal, B. Comparison of SO2 and H2SO4 impregnation of softwood prior to steam pretreatment on ethanol production. Appl. Biochem. Biotechnol 1998, 70, 3–15. [Google Scholar]
- Eklund, R; Galbe, M; Zacchi, G. The influence of SO2 and H2SO4 impregnation of willow prior to steam pretreatment. Bioresource Technol 1995, 52, 225–229. [Google Scholar]
- Stenberg, K; Tengborg, C; Galbe, M; Zacchi, G. Optimisation of steam pretreatment of SO2-impregnated mixed softwoods for ethanol production. J. Chem. Technol. Biotechnol 1998, 71, 299–308. [Google Scholar]
- McMillan, JD. Pretreatment of lignocellulosic biomass. In Enzymatic Conversion of Biomass for Fuels Production; Himmel, ME, Baker, JO, Overend, RP, Eds.; ACS: Washington DC, USA, 1994; pp. 292–324. [Google Scholar]
- Fan, L; Lee, Y; Gharpuray, M. The nature of lignocellulosics and their pretreatments for enzymatic hydrolysis. Adv. Biochem. Eng. Biotechnol 1982, 23, 158–183. [Google Scholar]
- Mais, U; Esteghlalian, AR; Saddler, JN; Mansfield, SD. Enhancing the enzymatic hydrolysis of cellulosic materials using simultaneous ball milling. Appl. Biochem. Biotechnol 2002, 98, 815–832. [Google Scholar]
- Tassinari, T; Macy, C. Differential speed two roll mill pretreatment of cellulosic materials for enzymatic hydrolysis. Biotechnol. Bioeng 1977, 19, 1321–1330. [Google Scholar]
- Zhang, RH; Zhang, ZQ. Biogasification of rice straw with an anaerobic-phased solids digester system. Bioresource Technol 1999, 68, 235–245. [Google Scholar]
- Muller, CD; Abu-Orf, M; Novak, JT. Application of mechanical shear in an internal-recycle for the enhancement of mesophilic anaerobic digestion. Water Environ. Res 2007, 79, 297–304. [Google Scholar]
- Walpot, JI. Enzymatic hydrolysis of waste paper. Conserv. Recycling 1986, 9, 127–136. [Google Scholar]
- Zeng, M; Mosier, NS; Huang, CP; Sherman, DM; Ladisch, MR. Microscopic examination of changes of plant cell structure in corn stover due to hot water pretreatment and enzymatic hydrolysis. Biotechnol. Bioeng 2007, 97, 265–278. [Google Scholar]
- Sidiras, DK; Koukios, EG. Acid saccharification of ball-milled straw. Biomass 1989, 19, 289–306. [Google Scholar]
- Ryu, SK; Lee, JM. Bioconversion of waste cellulose by using an attrition bioreactor. Biotechnol. Bioeng 1983, 25, 53–65. [Google Scholar]
- Sinitsyn, AP; Gusakov, AV; Davydkin, IY; Davydkin, VY; Protas, OV. A hyperefficient process for enzymatic cellulose hydrolysis in the intensive mass transfer reactor. Biotechnol. Lett 1993, 15, 283–288. [Google Scholar]
- Jin, S; Chen, H. Superfine grinding of steam-exploded rice straw and its enzymatic hydrolysis. Biochem. Eng. J 2006, 30, 225–230. [Google Scholar]
- Henley, RG; Yang, RYK; Greenfield, PF. Enzymatic saccharification of cellulose in membrane reactors. Enzyme Microb. Tech 1980, 2, 206–208. [Google Scholar]
- Mooney, CA; Mansfield, SD; Beatson, RP; Saddler, JN. The effect of fiber characteristics on hydrolysis and cellulase accessibility to softwood substrates. Enzyme Microb. Tech 1999, 25, 644–650. [Google Scholar]
- Kumakura, M; Kaetsu, I. Pretreatment by radiation and acids of chaff and its effect on enzymatic hydrolysis of cellulose. Agr. Wastes 1984, 9, 279–287. [Google Scholar]
- Mamar, SAS; Hadjadj, A. Radiation pretreatments of cellulose materials for the enhancement of enzymatic hydrolysis. Radiat. Phys. Chem 1990, 35, 451–455. [Google Scholar]
- Kumakura, M; Kaetsu, I. Effect of radiation pretreatment of bagasse on enzymatic and acid hydrolysis. Biomass 1983, 3, 199–208. [Google Scholar]
- Kumakura, M; Kaetsu, I. Radiation-induced decomposition and enzymatic hydrolysis of cellulose. Biotechnol. Bioeng 1978, 20, 1309–1315. [Google Scholar]
- Kumakura, M; Kaetsu, I. Radiation degradation and the subsequent enzymatic hydrolysis of waste papers. Biotechnol. Bioeng 1982, 24, 991–997. [Google Scholar]
- Kumakura, M; Kojima, T; Kaetsu, I. Pretreatment of lignocellulosic wastes by combination of irradiation and mechanical crushing. Biomass 1982, 2, 299–308. [Google Scholar]
- McDermott, BL; Chalmers, AD; Goodwin, JAS. Ultrasonication as a pre-treatment method for the enhancement of the psychrophilic anaerobic digestion of aquaculture effluents. Environ. Technol 2001, 22, 823–830. [Google Scholar]
- Wang, QH; Kuninobu, M; Ogawa, HI; Kato, Y. Degradation of volatile fatty acids in highly efficient anaerobic digestion. Biomass Bioenerg 1999, 16, 407–416. [Google Scholar]
- Cui, R; Jahng, D. Enhanced methane production from anaerobic digestion of disintegrated and deproteinized excess sludge. Biotechnol. Lett 2006, 28, 531–538. [Google Scholar]
- Chu, CP; Lee, DJ; Chang, BV; You, CS; Tay, JH. “Weak” ultrasonic pre-treatment on anaerobic digestion of flocculated activated biosolids. Water Res 2002, 36, 2681–2688. [Google Scholar]
- Wang, F; Wang, Y; Ji, M. Mechanisms and kinetics models for ultrasonic waste activated sludge disintegration. J. Hazard. Mater 2005, 123, 145–150. [Google Scholar]
- Dohanyos, M; Zabranska, J; Jenicek, P. Innovative technology for the improvement of the anaerobic methane fermentation. Water Sci. Technol 1997, 36, 333–340. [Google Scholar]
- Wang, Q; Noguchi, C; Hara, Y; Sharon, C; Kakimoto, K; Kato, Y. Studies on anaerobic digestion mechanism: Influence of pretreatment temperature on biodegradation of waste activated sludge. Environ. Technol 1997, 18, 999–1008. [Google Scholar]
- Lafitte-Trouque, S; Forster, CF. The use of ultrasound and gamma-irradiation as pre-treatments for the anaerobic digestion of waste activated sludge at mesophilic and thermophilic temperatures. Bioresource Technol 2002, 84, 113–118. [Google Scholar]
- Kennedy, KJ; Thibault, G; Droste, RL. Microwave enhanced digestion of aerobic SBR sludge. Water SA 2007, 33, 261–270. [Google Scholar]
- Park, B; Ahn, JH; Kim, J; Hwang, S. Use of microwave pretreatment for enhanced anaerobiosis of secondary sludge. Water Sci. Technol 2004, 50, 17–23. [Google Scholar]
- Eskicioglu, C; Terzian, N; Kennedy, KJ; Droste, RL; Hamoda, M. Athermal microwave effects for enhancing digestibility of waste activated sludge. Water Res 2007, 41, 2457–2466. [Google Scholar]
- Choi, H; Jeong, SW; Chung, YJ. Enhanced anaerobic gas production of waste activated sludge pretreated by pulse power technique. Bioresource Technol 2006, 97, 198–203. [Google Scholar]
- Chandra, R; Bura, R; Mabee, W; Berlin, A; Pan, X; Saddler, J. Substrate pretreatment: The key to effective enzymatic hydrolysis of lignocellulosics? Adv. Biochem. Eng. Biotechnol 2007, 108, 67–93. [Google Scholar]
- Varga, E; Reczey, K; Zacchi, G. Optimization of steam pretreatment of corn stover to enhance enzymatic digestibility. Appl. Biochem. Biotechnol 2004, 113, 509–523. [Google Scholar]
- Ruiz, E; Cara, C; Ballesteros, M; Manzanares, P; Ballesteros, I; Castro, E. Ethanol production from pretreated olive tree wood and sunflower stalks by an SSF process. Appl. Biochem. Biotechnol 2006, 129, 631–643. [Google Scholar]
- Kurabi, A; Berlin, A; Gilkes, N; Kilburn, D; Bura, R; Robinson, J; Markov, A; Skomarovsky, A; Gusakov, A; Okunev, O; Sinitsyn, A; Gregg, D; Xie, D; Saddler, J. Enzymatic hydrolysis of steam-exploded and ethanol organosolv-pretreated Douglas-Firby novel and commercial fungal cellulases. Appl. Biochem. Biotechnol 2005, 121, 219–230. [Google Scholar]
- Cullis, IF; Saddler, JN; Mansfield, SD. Effect of initial moisture content and chip size on the bioconversion efficiency of softwood lignocellulosics. Biotechnol. Bioeng 2004, 85, 413–421. [Google Scholar]
- Ballesteros, M; Oliva, JM; Negro, MJ; Manzanares, P; Ballesteros, I. Ethanol from lignocellulosic materials by a simultaneous saccharification and fermentation process (SFS) with Kluyveromyces marxianus CECT 10875. Process Biochem 2004, 39, 1843–1848. [Google Scholar]
- Josefsson, T; Lennholm, H; Gellerstedt, G. Steam explosion of aspen wood. Characterisation of reaction products. Holzforschung 2002, 56, 289–297. [Google Scholar]
- Ahring, BK; Thomsen, AB. A method for processing lignocellulosic material. European Patent EP1259466,. 2001. [Google Scholar]
- Carrasco, JE; Saiz, MC; Navarro, A; Soriano, P; Saez, F; Martinez, JM. Effects of dilute-acid and steam explosion pretreatments on the cellulose structure and kinetics of cellulosic fraction hydrolysis by dilute acids in lignocellulosic materials. Appl. Biochem. Biotechnol 1994, 45, 23–34. [Google Scholar]
- Mes-Hartree, M; Saddler, JN. The nature of inhibitory materials present in pretreated lignocellulosic substrates which inhibit the enzymic hydrolysis of cellulose. Biotechnol. Lett 1983, 5, 531–536. [Google Scholar]
- Pfeifer, PA; Bonn, G; Bobleter, O. Influence of biomass degradation products on the fermentation of glucose to ethanol by Saccharomyces carlsbergensis W 34. Biotechnol. Lett 1984, 6, 541–546. [Google Scholar]
- Laser, M; Schulman, D; Allen, SG; Lichwa, J; Antal, MJ, Jr; Lynd, LR. A comparison of liquid hot water and steam pretreatments of sugar cane bagasse for bioconversion to ethanol. Bioresource Technol 2002, 81, 33–44. [Google Scholar]
- Sun, XF; Xu, F; Sun, RC; Wang, YX; Fowler, P; Baird, MS. Characteristics of degraded lignins obtained from steam exploded wheat straw. Polym. Degrad. Stabil 2004, 86, 245–256. [Google Scholar]
- Ruiz, E; Cara, C; Manzanares, P; Ballesteros, M; Castro, E. Evaluation of steam explosion pre-treatment for enzymatic hydrolysis of sunflower stalks. Enzyme Microb. Tech 2008, 42, 160–166. [Google Scholar]
- Negro, MJ; Manzanares, P; Ballesteros, I; Oliva, JM; Cabanas, A; Ballesteros, M. Hydrothermal pretreatment conditions to enhance ethanol production from poplar biomass. Appl. Biochem. Biotechnol 2003, 105, 87–100. [Google Scholar]
- Mason, WH. Process and apparatus for disintegration of wood and the like. US Patent 1,578,609,. 1926. [Google Scholar]
- Katzen, R; Madson, PW; Monceaux, DA. Use of cellulosic feedstocks for alcohol production. In The Alcohols Textbook; Lyons, TP, Murtagh, JE, Kelsall, DR, Eds.; Nothingham University Press, 1995; pp. 37–46. [Google Scholar]
- Hooper, RJ; Li, J. Summary of the factors critical to the commercial application of bioenergy technologies. Biomass Bioenerg 1996, 11, 469–474. [Google Scholar]
- Ward, A; Stensel, HD; Ferguson, JF; Ma, G; Hummel, S. Effect of autothermal treatment on anaerobic digestion in the dual digestion process. Water Sci. Technol 1998, 38, 435–442. [Google Scholar]
- Bougrier, C; Delgenes, JP; Carrere, H. Impacts of thermal pre-treatments on the semi-continuous anaerobic digestion of waste activated sludge. Biochem. Eng. J 2007, 34, 20–27. [Google Scholar]
- Dereix, M; Parker, W; Kennedy, K. Steam-explosion pretreatment for enhancing anaerobic digestion of municipal wastewater sludge. Water Environ. Res 2006, 78, 474–485. [Google Scholar]
- Bougrier, C; Delgenes, JP; Carrere, H. Combination of thermal treatments and anaerobic digestion to reduce sewage sludge quantity and improve biogas yield. Process Saf. Environ. Protect 2006, 84, 280–284. [Google Scholar]
- Mladenovska, Z; Hartmann, H; Kvist, T; Sales-Cruz, M; Gani, R; Ahring, BK. Thermal pretreatment of the solid fraction of manure: impact on the biogas reactor performance and microbial community. Water Sci. Technol 2006, 53, 59–67. [Google Scholar]
- Solheim, OE. Method of and arrangement for continuous hydrolysis of organic material. US Patent 0,168,990,. 2004. [Google Scholar]
- Moeller-Chavez, G; Gonzalez-Martinez, S. Two combined techniques to enhance anaerobic digestion of sludge. Water Sci. Technol 2002, 46, 167–172. [Google Scholar]
- Kim, J; Park, C; Kim, TH; Lee, M; Kim, S; Kim, SW; Lee, J. Effects of various pretreatments for enhanced anaerobic digestion with waste activated sludge. J. Biosci. Bioeng 2003, 95, 271–275. [Google Scholar]
- DiStefano, TD; Ambulkar, A. Methane production and solids destruction in an anaerobic solid waste reactor due to post-reactor caustic and heat treatment. Water Sci. Technol 2006, 53, 33–41. [Google Scholar]
- Sun, XF; Xu, F; Sun, RC; Fowler, P; Bairdd, MS. Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohyd. Res 2005, 340, 97–106. [Google Scholar]
- Mosier, N; Wyman, C; Dale, B; Elander, R; Lee, YY; Holtzapple, M; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technol 2005, 96, 673–686. [Google Scholar]
- Chundawat, SP; Venkatesh, B; Dale, BE. Effect of particle size based separation of milled corn stover on AFEX pretreatment and enzymatic digestibility. Biotechnol. Bioeng 2007, 96, 219–231. [Google Scholar]
- Eggeman, T; Elander, RT. Process and economic analysis of pretreatment technologies. Bioresource Technol 2005, 96, 2019–2025. [Google Scholar]
- Zheng, Y; Tsao, GT. Avicel hydrolysis by cellulase enzyme in supercritical CO2. Biotechnol. Lett 1996, 18, 451–454. [Google Scholar]
- Zheng, Y; Lin, H-M; Wen, J; Cao, N; Yu, X; Tsao, GT. Supercritical carbon dioxide explosion as a pretreatment for cellulose hydrolysis. Biotechnol. Lett 1995, 17, 845–850. [Google Scholar]
- Kim, KH; Hong, J. Supercritical CO2 pretreatment of lignocellulose enhances enzymatic cellulose hydrolysis. Bioresource Technol 2001, 77, 139–144. [Google Scholar]
- Pasquini, D; Pimenta, MTB; Ferreira, LH; Curvelo, AAdS. Extraction of lignin from sugar cane bagasse and Pinus taeda wood chips using ethanol-water mixtures and carbon dioxide at high pressures. J. Supercrit. Fluid 2005, 36, 31–39. [Google Scholar]
- Park, CY; Ryu, YW; Kim, C. Kinetics and rate of enzymatic hydrolysis of cellulose in supercritical carbon dioxide. Korean J. Chem. Eng 2001, 18, 475–478. [Google Scholar]
- Dien, BS; Li, XL; Iten, LB; Jordan, DB; Nichols, NN; O’Bryan, PJ; Cotta, MA. Enzymatic saccharification of hot-water pretreated corn fiber for production of monosaccharides. Enzyme Microb. Tech 2006, 39, 1137–1144. [Google Scholar]
- Sreenath, HK; Koegel, RG; Moldes, AB; Jeffries, TW; Straub, RJ. Enzymic saccharification of alfalfa fibre after liquid hot water pretreatment. Process Biochem 1999, 35, 33–41. [Google Scholar]
- Mosier, N; Hendrickson, R; Ho, N; Sedlak, M; Ladisch, MR. Optimization of pH controlled liquid hot water pretreatment of corn stover. Bioresource Technol 2005, 96, 1986–1993. [Google Scholar]
- Mosier, NS; Hendrickson, R; Brewer, M; Ho, N; Sedlak, M; Dreshel, R; Welch, G; Dien, BS; Aden, A; Ladisch, MR. Industrial scale-up of pH-controlled liquid hot water pretreatment of corn fiber for fuel ethanol production. Appl. Biochem. Biotechnol 2005, 125, 77–97. [Google Scholar]
- Kim, TH; Lee, YY. Fractionation of corn stover by hot-water and aqueous ammonia treatment. Bioresource Technol 2006, 97, 224–232. [Google Scholar]
- Zhu, S; Wu, Y; Yu, Z; Liao, J; Zhang, Y. Pretreatment by microwave/alkali of rice straw and its enzymatic hydrolysis. Process Biochem 2005, 40, 3082–3086. [Google Scholar]
- Zhu, S; Wu, Y; Yu, Z; Wang, C; Yu, F; Jin, S; Ding, Y; Chi, R; Liao, J; Zhang, Y. Comparison of three microwave/chemical pretreatment processes for enzymatic hydrolysis of rice straw. Biosyst. Eng 2006, 93, 279–283. [Google Scholar]
- Kassim, EA; El-Shahed, AS. Enzymatic and chemical hydrolysis of certain cellulosic materials. Agr. Wastes 1986, 17, 229–233. [Google Scholar]
- Xu, Z; Wang, Q; Jiang, Z; Yang, X-X; Ji, Y. Enzymatic hydrolysis of pretreated soybean straw. Biomass Bioenerg 2007, 31, 162–167. [Google Scholar]
- Vaccarino, C; Lo Curto, RB; Tripodo, MM; Bellocco, E; Laganfi, G; Patan, R. Effect of SO2, NaOH and Na2CO3 pretreatments on the degradability and cellulase digestibility of grape marc. Biol. Waste 1987, 20, 79–88. [Google Scholar]
- Silverstein, RA; Chen, Y; Sharma-Shivappa, RR; Boyette, MD; Osborne, J. A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresource Technol 2007, 98, 3000–3011. [Google Scholar]
- Zhao, X; Zhang, L; Liu, D. Comparative study on chemical pretreatment methods for improving enzymatic digestibility of crofton weed stem. Bioresource Technol 2007, 99, 3729–3736. [Google Scholar]
- Gaspar, M; Kalman, G; Reczey, K. Corn fiber as a raw material for hemicellulose and ethanol production. Process Biochem 2007, 42, 1135–1139. [Google Scholar]
- Beccari, M; Majone, M; Papini, MP; Torrisi, L. Enhancement of anaerobic treatability of olive oil mill effluents by addition of Ca(OH)2 and bentonite without intermediate solid/liquid separation. Water Sci. Technol 2001, 43, 275–282. [Google Scholar]
- Tanaka, S; Kobayashi, T; Kamiyama, K; Bildan, M. Effects of thermochemical pretreatment on the anaerobic digestion of waste activated sludge. Water Sci. Technol 1997, 35, 209–215. [Google Scholar]
- Tanaka, S; Kamiyama, K. Thermochemical pretreatment in the anaerobic digestion of waste activated sludge. Water Sci. Technol 2002, 46, 173–179. [Google Scholar]
- Lin, JG; Ma, YS; Chao, AC; Huang, CL. BMP test on chemically pretreated sludge. Bioresource Technol 1999, 68, 187–192. [Google Scholar]
- Heo, NH; Park, SC; Lee, JS; Kang, H. Solubilization of waste activated sludge by alkaline pretreatment and biochemical methane potential (BMP) tests for anaerobic co-digestion of municipal organic waste. Water Sci. Technol 2003, 48, 211–219. [Google Scholar]
- Saha, BC; Cotta, MA. Ethanol production from alkaline peroxide pretreated enzymatically saccharified wheat straw. Biotechnol. Progr 2006, 22, 449–453. [Google Scholar]
- Saha, BC; Cotta, MA. Enzymatic saccharification and fermentation of alkaline peroxide pretreated rice hulls to ethanol. Enzyme Microb. Tech 2007, 41, 528–532. [Google Scholar]
- Mishima, D; Tateda, M; Ike, M; Fujita, M. Comparative study on chemical pretreatments to accelerate enzymatic hydrolysis of aquatic macrophyte biomass used in water purification processes. Bioresource Technol 2006, 97, 2166–2172. [Google Scholar]
- Curreli, N; Fadda, MB; Rescigno, A; Rinaldi, AC; Soddu, G; Sollai, F; Vaccargiu, S; Sanjust, E; Rinaldi, A. Mild alkaline/oxidative pretreatment of wheat straw. Process Biochem 1997, 32, 665–670. [Google Scholar]
- Itoh, H; Wada, M; Honda, Y; Kuwahara, M; Watanabe, T. Bioorganosolve pretreatments for simultaneous saccharification and fermentation of beech wood by ethanolysis and white rot fungi. J. Biotechnol 2003, 103, 273–280. [Google Scholar]
- Pan, X; Gilkes, N; Kadla, J; Pye, K; Saka, S; Gregg, D; Ehara, K; Xie, D; Lam, D; Saddler, J. Bioconversion of hybrid poplar to ethanol and co-products using an organosolv fractionation process: optimization of process yields. Biotechnol. Bioeng 2006, 94, 851–861. [Google Scholar]
- Rolz, C; de Arriola, MC; Valladares, J; de Cabrera, S. Effects of some physical and chemical pretreatments on the composition and enzymatic hydrolysis and digestibility of lemon grass and citronella bagasse. Agr. Wastes 1986, 18, 145–161. [Google Scholar]
- Pan, X; Arato, C; Gilkes, N; Gregg, D; Mabee, W; Pye, K; Xiao, Z; Zhang, X; Saddler, J. Biorefining of softwoods using ethanol organosolv pulping: Preliminary evaluation of process streams for manufacture of fuel-grade ethanol and co-products. Biotechnol. Bioeng 2005, 90, 473–481. [Google Scholar]
- Araque, E; Parra, C; Freer, J; Contreras, D; Rodriguez, J; Mendonca, R; Baeza, J. Evaluation of organosolv pretreatment for the conversion of Pinus radiata D. Don to ethanol. Enzyme Microb. Tech 2007, 43, 214–219. [Google Scholar]
- Papatheofanous, MG; Billa, E; Koullas, DP; Monties, B; Koukios, EG. Two-stage acid-catalyzed fractionation of lignocellulosic biomass in aqueous ethanol systems at low temperatures. Bioresource Technol 1995, 54, 305–310. [Google Scholar]
- Palonen, H; Thomsen, AB; Tenkanen, M; Schmidt, AS; Viikari, L. Evaluation of wet oxidation pretreatment for enzymatic hydrolysis of softwood. Appl. Biochem. Biotechnol 2004, 117, 1–17. [Google Scholar]
- Varga, E; Klinke, HB; Reczey, K; Thomsen, AB. High Solid Simultaneous Saccharification and Fermentation of Wet Oxidized Corn Stover to Ethanol. Biotechnol. Bioeng 2004, 88, 567–574. [Google Scholar]
- Garrote, G; Dominguez, H; Parajo, JC. Hydrothermal processing of lignocellulosic materials. Holz Als Roh-und Werkst 1999, 57, 191–202. [Google Scholar]
- Schmidt, A; Thomsen, A. Optimization of wet oxidation pretreatment of wheat straw. Bioresource Technol 1998, 64, 139–151. [Google Scholar]
- Saha, BC. Hemicellulose bioconversion. Ind. Microbiol. Biotechnol 2003, 30, 279–291. [Google Scholar]
- Schultz, TP; McGinnis, GD; Biermann, CJ. Similarities and differences in pretreating woody biomass by steam explosion, wet oxidation, autohydrolysis, and rapid steam hydrolysis/continuous extraction. Proceedings of Annual Symposium on Energy from Biomass and Wastes, Lake Buena Vista, FL, USA; 1984. [Google Scholar]
- Bjerre, AB; Olesen, AB; Fernqvist, T. Pretreatment of wheat straw using combined wet oxidation and alkaline hydrolysis resulting in convertible cellulose and hemicellulose. Biotechnol. Bioeng 1996, 49, 568–577. [Google Scholar]
- Ahring, BK; Jensen, K; Nielsen, P; Bjerre, AB; Schmidt, AS. Pretreatment of wheat straw and conversion of xylose and xylan to ethanol by thermophilic anaerobic bacteria. Bioresource Technol 1996, 58, 107–113. [Google Scholar]
- Martin, C; Klinke, HB; Thomsen, AB. Wet oxidation as a pretreatment method for enhancing the enzymatic convertibility of sugarcane bagasse. Enzyme Microb. Tech 2007, 40, 426–432. [Google Scholar]
- Lissens, G; Thomsen, AB; De Baere, L; Verstraete, W; Ahring, BK. Thermal wet oxidation improves anaerobic biodegradability of raw and digested biowaste. Environ. Sci. Technol 2004, 38, 3418–3424. [Google Scholar]
- Galbe, M; Zacchi, G. A review of the production of ethanol from softwood. Appl. Microbiol. Biotechnol 2002, 59, 618–628. [Google Scholar]
- Azzam, AM. Pretreatment of cane bagasse with alkaline hydrogen peroxide for enzymatic hydrolysis of cellulose and ethanol fermentation. J. Environ. Sci. Heal 1989, 24, 421–433. [Google Scholar]
- Vidal, PF; Molinier, J. Ozonolysis of Lignin – Improvement of in vitro digestibility of poplar sawdust. Biomass 1988, 16, 1–17. [Google Scholar]
- Neely, WC. Factors affecting the pretreatment of biomass with gaseous ozone. Biotechnol. Bioeng 1984, 26, 59–65. [Google Scholar]
- Weemaes, M; Grootaerd, H; Simoens, F; Verstraete, W. Anaerobic digestion of ozonized biosolids. Water Res 2000, 34, 2330–2336. [Google Scholar]
- Goel, R; Tokutomi, T; Yasui, H. Anaerobic digestion of excess activated sludge with ozone pretreatment. Water Sci. Technol 2003, 47, 207–214. [Google Scholar]
- Goel, R; Tokutomi, T; Yasui, H; Noike, T. Optimal process configuration for anaerobic digestion with ozonation. Water Sci. Technol 2003, 48, 85–96. [Google Scholar]
- Benitez, FJ; BeltranHeredia, J; Torregrosa, J; Acero, JL. Improvement of the anaerobic biodegradation of olive mill wastewaters by prior ozonation pretreatment. Bioprocess Eng 1997, 17, 169–175. [Google Scholar]
- Jones, J; Semrau, K. Wood hydrolysis for ethanol production previous experience and the economics of selected processes. Biomass 1984, 5, 109–135. [Google Scholar]
- Yang, B; Wyman, CE. Effect of xylan and lignin removal by batch and flowthrough pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnol. Bioeng 2004, 86, 88–95. [Google Scholar]
- Sun, Y; Cheng, JJ. Dilute acid pretreatment of rye straw and bermudagrass for ethanol production. Bioresource Technol 2005, 96, 1599–606. [Google Scholar]
- Cara, C; Ruiz, E; Oliva, JM; Saez, F; Castro, E. Conversion of olive tree biomass into fermentable sugars by dilute acid pretreatment and enzymatic saccharification. Bioresource Technol 2007, 99, 1869–1876. [Google Scholar]
- Xiao, WP; Clarkson, WW. Acid solubilization of lignin and bioconversion of treated newsprint to methane. Biodegradation 1997, 8, 61–66. [Google Scholar]
- Chen, YG; Jiang, S; Yuan, HY; Zhou, Q; Gu, GW. Hydrolysis and acidification of waste activated sludge at different pHs. Water Res 2007, 41, 683–689. [Google Scholar]
- Kivaisi, AK; Eliapenda, S. Pretreatment of bagasse and coconut fibres for enhanced anaerobic degradation by rumen microorganisms. Renew. Energy 1994, 5, 791–795. [Google Scholar]
- Azzam, AM. Saccharification of bagasse cellulose pretreated with ZnCl2 and HCl. Biomass Bioenerg 1987, 12, 71–77. [Google Scholar]
- Taniguchi, M; Suzuki, H; Watanabe, D; Sakai, K; Hoshino, K; Tanaka, T. Evaluation of pretreatment with Pleurotus ostreatus for enzymatic hydrolysis of rice straw. J. Biosci. Bioeng 2005, 100, 637–43. [Google Scholar]
- Kurakake, M; Ide, N; Komaki, T. Biological pretreatment with two bacterial strains for enzymatic hydrolysis of office paper. Curr Microbiol 2007, 54, 424–428. [Google Scholar]
- Srilatha, HR; Nand, K; Babu, KS; Madhukara, K. Fungal pretreatment of orange processing wastes by solid-state fermentation for improved production of methane. Process Biochem 1995, 30, 327–331. [Google Scholar]
- Dhouib, A; Ellouz, M; Aloui, F; Sayadi, S. Effect of bioaugmentation of activated sludge with white-rot fungi on olive mill wastewater detoxification. Lett. Appl. Microbiol 2006, 42, 405–411. [Google Scholar]
| Pretreatment method | Processes | Studied application | Possible changes in biomass | Notable remarks | Selected References |
|---|---|---|---|---|---|
| Physical pretreatments | Milling: - Ball milling - Two-roll milling - Hammer milling - Colloid illing - Vibro energy milling | Ethanol | - Increase in accessible surface area and pore size
- Decrease in cellulose crystallinity - Decrease in degrees of polymerization | - Most of the methods are highly energy-demanding
- Most of them cannot remove the lignin - It is preferable not to use these methods for industrial applications - No chemicals are generally required for these methods | [57, 58, 63] |
| Irradiation: - Gamma-ray irradiation - Electron-beam irradiation - Microwave irradiation | Ethanol and biogas | [74, 82–85] | |||
| Others: - Hydrothermal - High pressure steaming - Expansion - Extrusion - Pyrolysis | Ethanol and biogas | [101, 153] | |||
| Chemical and physicochemical pretreatments | Explosion: - Steam explosion - Ammonia fiber explosion (AFEX) - CO2 explosion - SO2 explosion | Ethanol and biogas | [15, 37, 43, 46, 47, 50–54, 93, 95, 100, 118–120, 156] | ||
| Alkali: - Sodium hydroxide - Ammonia - Ammonium Sulfite | Ethanol and biogas | [132, 127] | |||
| Acid: - Sulfuric acid - Hydrochloric acid - Phosphoric acid | Ethanol and biogas | - Increase in accessible surface area
- Partial or nearly complete delignification - Decrease in cellulose crystallinity - Decrease in degrees of polymerization - Partial or complete hydrolysis of hemicelluloses | - These methods are among the most effective and include the most promising processes for industrial applications
- Usually rapid treatment rate - Typically need harsh conditions - There are chemical requirements | [3, 4, 21, 36] | |
| Gas: - Chlorine dioxide - Nitrogen dioxide - Sulfur dioxide | Ethanol and biogas | [56] | |||
| Oxidizing agents: - Hydrogen peroxide - Wet oxidation - Ozone | Ethanol and biogas | [151, 154, 156, 157, 159, 162, 164–168] | |||
| Solvent extraction of lignin: - Ethanol-water extraction - Benzene-water extraction - Ethylene glycol extraction - Butanol-water extraction - Swelling agents | Ethanol
| [121]
| |||
| Biological pretreatments | Fungi and actinomycetes | Ethanol and biogas | - Delignification
- Reduction in degree of polymerization of cellulose - Partial hydrolysis of hemicellulose | - Low energy requirement
- No chemical requirement - Mild environmental conditions - Very low treatment rate - Did not consider for commercial application | [158, 178–180] |
© 2008 by MDPI This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621-1651. https://doi.org/10.3390/ijms9091621
Taherzadeh MJ, Karimi K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. International Journal of Molecular Sciences. 2008; 9(9):1621-1651. https://doi.org/10.3390/ijms9091621
Chicago/Turabian StyleTaherzadeh, Mohammad J., and Keikhosro Karimi. 2008. "Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review" International Journal of Molecular Sciences 9, no. 9: 1621-1651. https://doi.org/10.3390/ijms9091621
APA StyleTaherzadeh, M. J., & Karimi, K. (2008). Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. International Journal of Molecular Sciences, 9(9), 1621-1651. https://doi.org/10.3390/ijms9091621






