Synthesis and Characterization of Polyacetylene with Side-chain Thiophene Functionality
Abstract
:Introduction
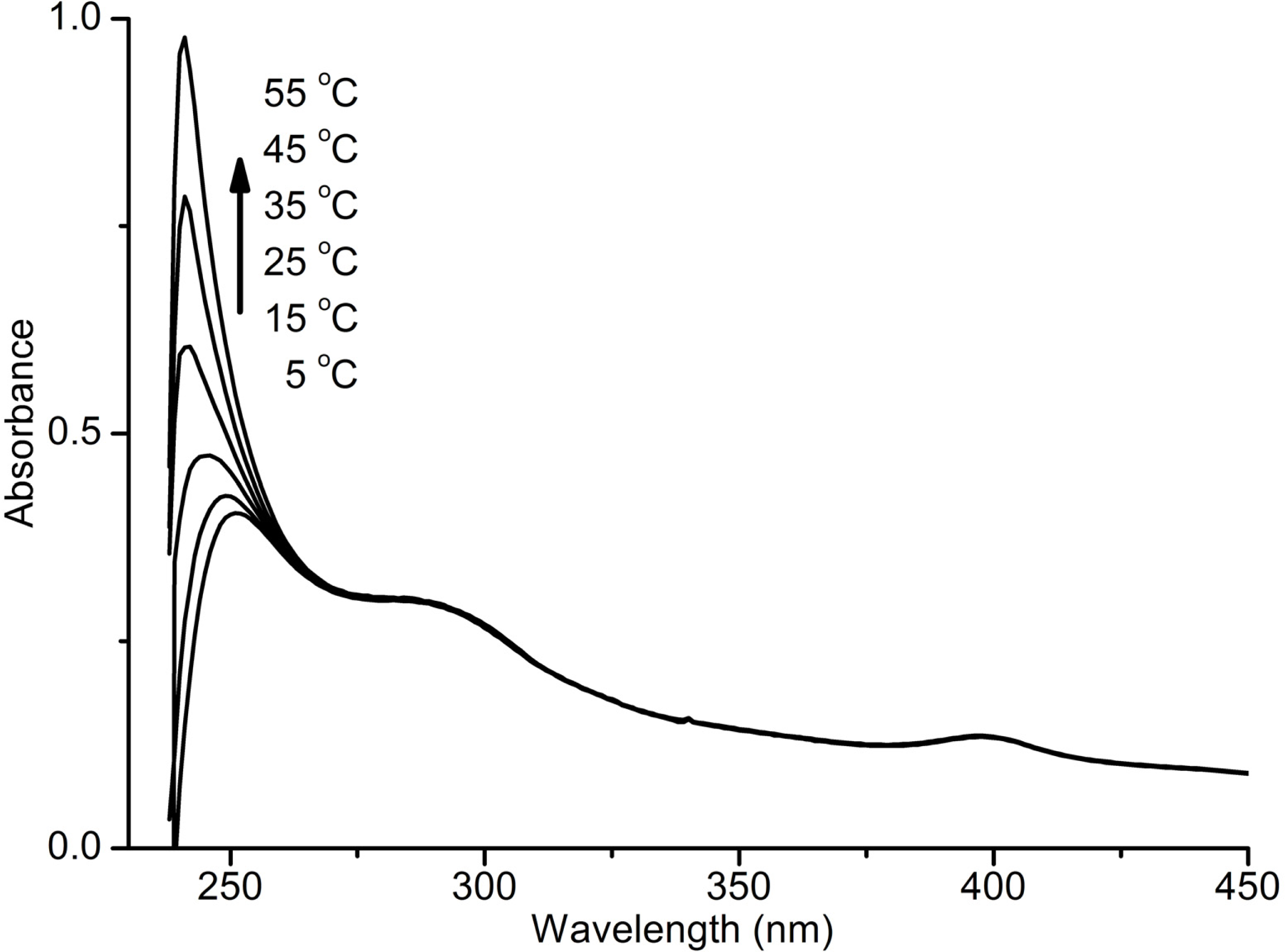
Results and Discussions
Experimental Section
Materials
Characterization
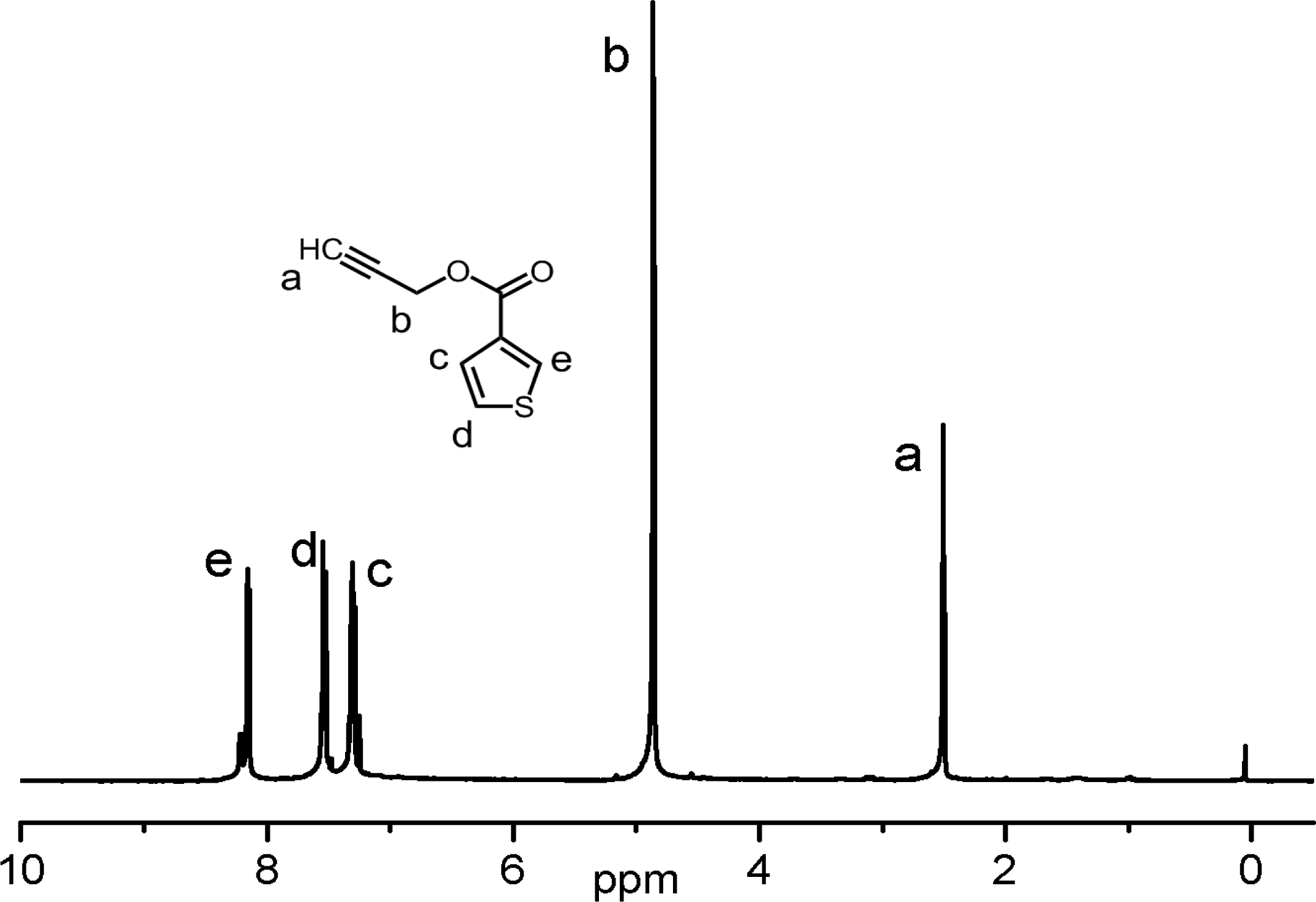
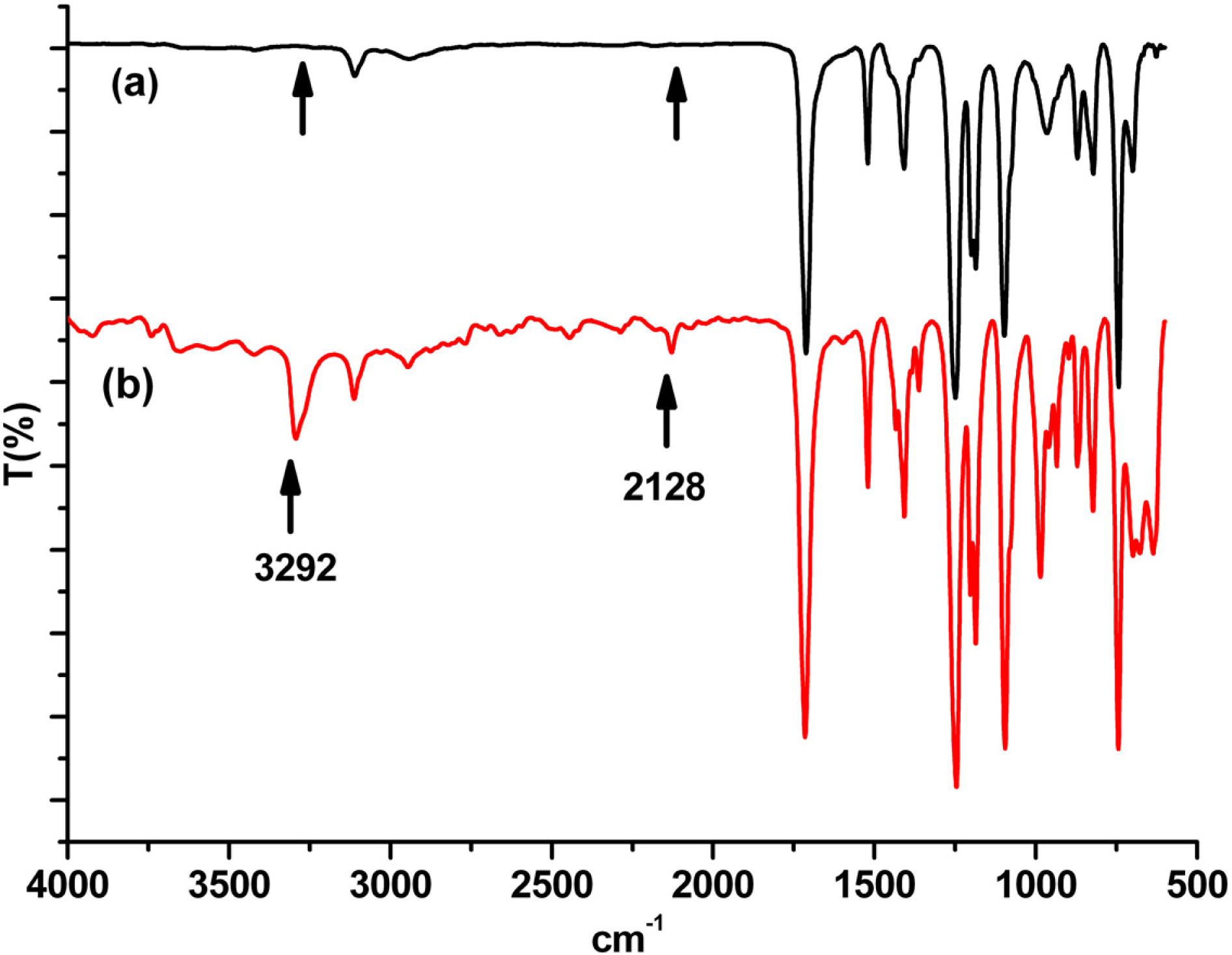
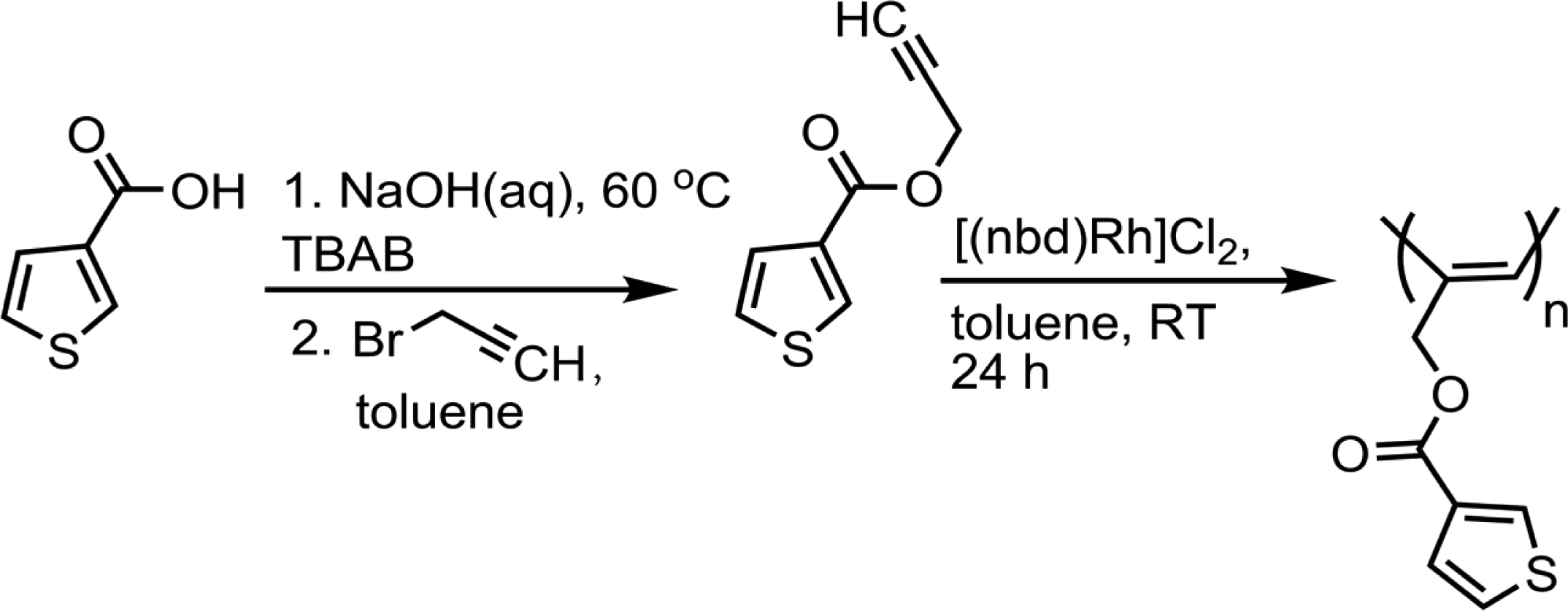
Monomer synthesis
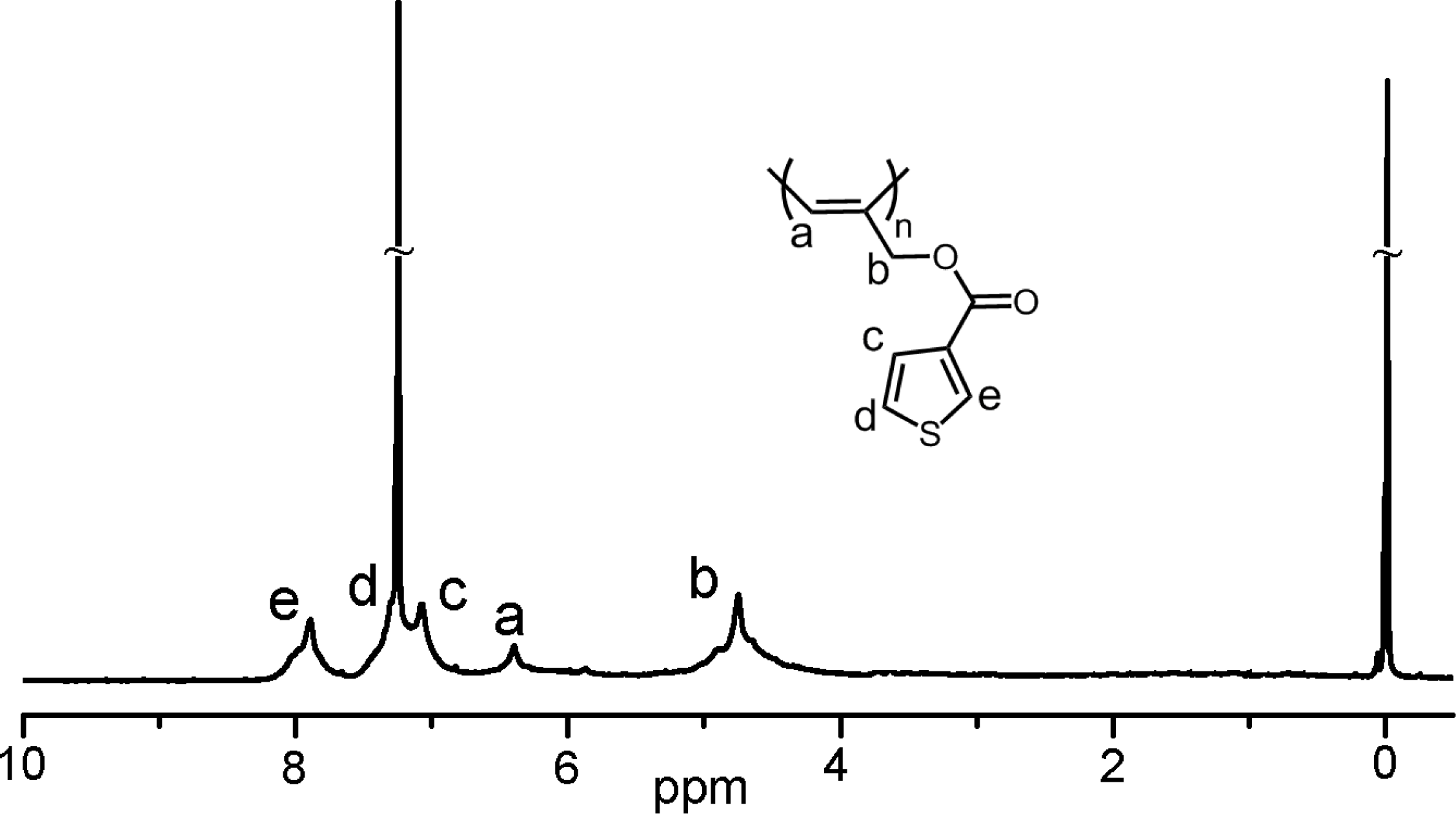
Polymerization
Acknowledgments
References
- Masuda, T; Sanda, F. Polymerization of substituted acetylenes. Handbook of metathesis 2003, 3, Chapter 11. 375. [Google Scholar] [Green Version]
- Sedlacek, J; Vohlidal, J. Controlled and living polymerizations induced with rhodium catalysts. Collect Czech Chem Commun 2003, 68, 1745–1790. [Google Scholar] [Green Version]
- Choi, S-K; Gal, Y-S; Jin, S-H; Kim, HK. Poly(1,6-heptadiyne)-based materials by metathesis polymerization. Chem Rev 2000, 100, 1645–1682. [Google Scholar] [Green Version]
- Tabata, M; Sone, T; Sadahiro, Y. Precise synthesis of monosubstituted polyacetylenes using Rh complex catalysts. Control of solid structure and π-conjugation length. Macromol Chem Phys 1999, 200, 265–282. [Google Scholar] [Green Version]
- Furlani, A; Napoletano, C; Russo, MV; Camus, A; Marsich, N. The influence of the ligands on the catalytic activity of a series of RhI complexes in reactions with phenylacetylene: Synthesis of stereoregular poly(phenyl) acetylene. J Polym Sci, Part A: Polym Chem 1989, 27, 75–86. [Google Scholar] [Green Version]
- Furlani, A; Napoletano, C; Russo, MV; Feast, WJ. Stereoregular polyphenylacetylene. Polym Bull 1986, 16, 311–317. [Google Scholar] [Green Version]
- Tabata, M; Yang, W; Yokota, K. 1H-NMR and UV studies of Rh complexes as a stereoregular polymerization catalysts for phenylacetylenes: Effects of ligands and solvents on its catalyst activity. J Polym Sci, Part A: Polym Chem 1994, 32, 1113–1120. [Google Scholar] [Green Version]
- Tabata, M; Yang, W; Yokota, K. Polymerization of m-chlorophenylacetylene initiated by [Rh(norbornadiene)Cl]2-triethylamine catalyst containing long-lived propagation species. Polym. J. 1990, 22, 1105–1107. [Google Scholar] [Green Version]
- Mastrorilli, P; Nobile, CF; Gallo, V; Suranna, GP; Farinola, G. Rhodium(I) catalyzed polymerization of phenylacetylene in ionic liquids. J. Mol. Catal. A: Chem. 2002, 184, 73–78. [Google Scholar] [Green Version]
- Tang, BZ; Poon, WH; Leung, SM; Leung, WH; Peng, H. Synthesis of stereoregular poly(phenylacetylene)s by organorhodium complexes in aqueous Media. Macromolecules 1997, 30, 2209–2212. [Google Scholar] [Green Version]
- Kishimoto, Y; Itou, M; Miyatake, Y; Ikariya, T; Noyori, R. Polymerization of monosubstituted acetylenes with a zwitterionic rhodium(I) complex, Rh+(2,5-norbornadiene)[.eta.6-C6H5)B-(C6H5)3]. Macromolecules 1995, 28, 6662–6666. [Google Scholar] [Green Version]
- Aoki, T; Kokai, M; Shinohara, K; Oikawa, E. Chiral helical conformation of the polyphenylacetylene having optically-active bulky substituent. Chem. Lett. 1993, 22, 2009. [Google Scholar] [Green Version]
- Kozuka, M; Sone, T; Sadahiro, Y; Tabata, M; Enoto, T. Columnar. Assemblies of Aliphatic Poly(acetylene ester)s prepared with a [Rh(norbornadiene)Cl]2 Catalyst. 1H and 13C NMR, X-Ray Diffraction and AFM Studies. Macromol. Chem. Phys. 2002, 203, 66–70. [Google Scholar] [Green Version]
- Tabata, M; Inaba, Y; Yokota, K; Nozaki, Y. Stereoregular polymerization of alkyl propiolate catalyzed by Rh complex. J. Macromol. Sci., Pure Appl. Chem. 1994, A31, 465–475. [Google Scholar] [Green Version]
- Nakako, H; Nomura, R; Masuda, T. Helix inversion of poly(propiolic esters). Macromolecules 2001, 34, 1496–1502. [Google Scholar] [Green Version]
- Nakako, H; Mayahara, Y; Nomura, R; Tabata, M; Masuda, T. Effect of chiral substituents on the helical conformation of poly(propiolic esters). Macromolecules 2000, 33, 3978–3982. [Google Scholar] [Green Version]
- Nomura, R; Fukushima, Y; Nakako, H; Masuda, T. Conformational study of helical poly(propiolic esters) in solution. J. Am. Chem. Soc. 2000, 122, 8830–8836. [Google Scholar] [Green Version]
- Nakako, H; Nomura, R; Tabata, M; Masuda, T. Synthesis and structure in solution of poly[(-)-menthyl propiolate] as a new class of helical polyacetylene. Macromolecules 1999, 32, 2861–2864. [Google Scholar] [Green Version]
- Tabei, J; Nomura, R; Masuda, T. Synthesis and structure of poly(N-propargylbenzamides) bearing chiral ester groups. Macromolecules 2003, 36, 573–577. [Google Scholar] [Green Version]
- Nomura, R; Tabei, J; Masuda, T. Effect of side chain structure on the conformation of poly(N-propargylalkylamide). Macromolecules 2002, 35, 2955–2961. [Google Scholar] [Green Version]
- Tabei, J; Nomura, R; Masuda, T. Conformational study of poly(N-propargylamides) having bulky pendant groups. Macromolecules 2002, 35, 5405–5409. [Google Scholar] [Green Version]
- Nomura, R; Tabei, J; Masuda, T. Biomimetic stabilization of helical structure in a synthetic polymer by means of intramolecular hydrogen bonds. J. Am. Chem. Soc. 2001, 123, 8430–8431. [Google Scholar] [Green Version]
- Yashima, E; Matsushima, T; Okamoto, Y. Chirality assignment of amines and amino alcohols based on circular dichroism induced by helix formation of a stereoregular poly((4-carboxyphenyl)acetylene) through acid-base complexation. J. Am. Chem. Soc. 1997, 119, 6345–6359. [Google Scholar] [Green Version]
- Yashima, E; Oobo, M; Nonokawa, R. Helicity induction on a poly(phenylacetylene) derivative bearing aza-15-crown-5 ether pendants in organic solvents and water. Macromolecules 2003, 36, 6599–6606. [Google Scholar] [Green Version]
- Yashima, E; Zhang, H-Q; Goto, H. Chiral stimuli-responsive gels: Helicity induction in poly(phenylacetylene) gels bearing a carboxyl group with chiral amines. J. Am. Chem. Soc. 2003, 125, 2516–2523. [Google Scholar] [Green Version]
- Yashima, E; Maeda, K; Sato, T; Okamoto, Y; Morini, K. Mechanism of helix induction on a stereoregular poly((4-carboxyphenyl)acetylene) with chiral amines and memory of the macromolecular helicity assisted by interaction with achiral amines. J. Am. Chem. Soc. 2004, 126, 4329–4342. [Google Scholar] [Green Version]
- Masuda, T; Sanda, F; Shiotsuki, M. Polymerization of acetylenes. Comprehensive Organometallic Chemistry III 2006, 11. Chapter 18. [Google Scholar]
- Aoki, T; Kaneko, T; Teraguchi, M. Synthesis of functional π-conjugated polymers from aromatic acetylenes. Polymer 2006, 47, 4867–4892. [Google Scholar] [Green Version]
- Lam, JWY; Tang, BZ. Functional Polyacetylenes. Acc. Chem Res. 2005, 38, 745–754. [Google Scholar] [Green Version]
- Masuda, T; Higashimura, T. Polyacetylenes with substituents: Their synthesis and properties. Adv. Polym. Sci. 1987, 81, 121–165. [Google Scholar] [Green Version]
- Gibson, HW; Pochan, JM. Concise encyclopedia of polymer science and engineering; Kroschwitz, JI, Ed.; Wiley: New York, NY, 1990; pp. 7–9. [Google Scholar]
- Ginsburg, EJ; Gorman, CB; Grubbs, RH. Modern acetylene chemistry; Stang, PJ, Diederich, F, Eds.; VCH: New York, NY, 1995; Chapter 10; pp. 353–383. [Google Scholar]
- Reddinger, JL; Reynolds, JR. Molecular Engineering of p-Conjugated Polymers. Adv. Polym. Sci. 1999, 145, 57–122. [Google Scholar] [Green Version]
- Volidal, J; Sedlacek, J. Chromatography of polymers: Hyphenated and multidimensional techniques; ACS Symposium Series 731; Provder, T, Ed.; American Chemical Society: Washington, DC, 1999; Chapter 19; p. 263. [Google Scholar]
- Masuda, T; Tang, BZ; Higashimura, T. Thermal degradation of polyacetylenes carrying substituents. Macromolecules 1985, 18, 2369–2373. [Google Scholar] [Green Version]
- Masuda, T; Tang, BZ; Tanaka, T; Higashimura, T. Mechanical properties of substituted polyacetylenes. Macromolecules 1986, 19, 1459–1464. [Google Scholar] [Green Version]
- Seki, H; Tang, BZ; Tanaka, A; Masuda, T. Tensile and dynamic viscoelastic properties of various new substituted polyacetylenes. Polymer 1994, 35, 3456–3462. [Google Scholar] [Green Version]
- Karim, SM; Nomura, R; Masuda, T. Degradation behavior of stereoregular cis-transoidal poly(phenylacetylene)s. J. Polym. Sci., Part A: Polym Chem. 2001, 39, 3130–3136. [Google Scholar] [Green Version]
- Hong, XM; Collard, DM. Liquid crystalline regioregular semifluoroalkyl-substituted polythiophenes. Macromolecules 2000, 33, 6916–6917. [Google Scholar] [Green Version]
- Goto, H. Cholesteric liquid crystal inductive asymmetric polymerization: Synthesis of chiral polythiophene derivatives from achiral monomers in a cholesteric liquid crystal. Macromolecules 2007, 40, 1377–1385. [Google Scholar] [Green Version]
- Yagci, Y; Toppare, L. Electroactive macromonomers based on pyrrole and thiophene: A versatile route to conducting block and graft copolymers. Polym. Int. 2003, 52, 1573–1578. [Google Scholar] [Green Version]
- Yilmaz, F; Guner, Y; Toppare, L; Yagci, Y. Synthesis and characterization of alternating copolymers of thiophene containing N- phenyl maleimide and styrene via photo-induced radical polymerization and their use in electropolymerization. Polymer 2004, 45, 5765–5774. [Google Scholar] [Green Version]
- Cianga, L; Yagci, Y. Synthesis and characterization of poly(N-phenyl maleimide) polymers with pendant thiophene rings by photoinduced radical polymerization. Polym. Sci., Polym. Chem. Ed. 2002, 15, 995–1004. [Google Scholar] [Green Version]
- Yagci, Y; Toppare, L. Synthesis of conducting block and graf copolymers containing polyether segments. Macromol. Symp. 2000, 157, 29–38. [Google Scholar] [Green Version]
- Oztemiz, S; Toppare, L; Onen, A; Yagci, Y. Conducting multiphase block copolymers of pyrrole with polytetrahydrofuran and polyetrahydrofuran-b-polystyrene. J. Macromol. Sci. 2000, A37, 277–291. [Google Scholar] [Green Version]
- Alkan, S; Toppare, L; Hepuzer, Y; Yagci, Y. Block copolymers of thiophene-capped poly(methyl methacrylate) with pyrrole. J. Polym. Sci., Polym. Chem. Ed. 1999, 37, 4218–4225. [Google Scholar] [Green Version]
- Alkan, S; Toppare, L; Hepuzer, Y; Yagci, Y. Synthesis and characterization of conducting block copolymers of thiophene-ended polystyrene with pyrrole. Synt. Met. 2001, 119, 133–134. [Google Scholar] [Green Version]
- Cirpan, A; Alkan, S; Toppare, L; Hepuzer, Y; Yagci, Y. Conducting graft copolymers of poly(3-methyl thienyl methacrylate) with pyrrole and thiophene. J. Polym. Sci., Polym. Chem. Ed. 2002, 40, 4131–4140. [Google Scholar] [Green Version]
- Kanki, K; Misumi, Y; Masuda, T. Remarkable cocatalytic effect of organometallics and rate control by triphenylphosphine in the Rh-catalyzed polymerization of phenylacetylene. Macromolecules 1999, 32, 2384–2386. [Google Scholar] [Green Version]
- Nakazato, A; Saeed, I; Katsumata, T; Shiotsuki, M; Masuda, T; Zednik, J; Vohlidal, J. Polymerization of Substituted Acetylenes by Various Rhodium Catalysts: Comparison of Catalyst Activity and Effect of Additives. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4530–4536. [Google Scholar] [Green Version]
- Koepp, HM; Wendt, H; Strehlow, HZ. Der vergleich der spannungsreihen in verschiedenen solventien II. Elektrochem. 1960, 64, 483. [Google Scholar] [Green Version]
- Bredas, JL; Silbey, R; Bourdreaux, DS; Chance, RR. Chain-length dependence of electronic and electrochemical properties of conjugated systems: polyacetylene, polyphenylene, polythiophene, and polpyrrole. J. Am. Chem. Soc. 1983, 105, 6555–6559. [Google Scholar] [Green Version]
- Nakamura, M; Tabata, M; Sone, T; Mawatari, Y; Miyasaka, A. Photoinduced cis-to-trans isomerization of poly(2-ethynylthiophene) prepared with a [Rh(norbornadiene)Cl]2 catalyst.1H NMR, UV, and ESR studies. Macromolecules 2002, 35, 200–2004. [Google Scholar] [Green Version]
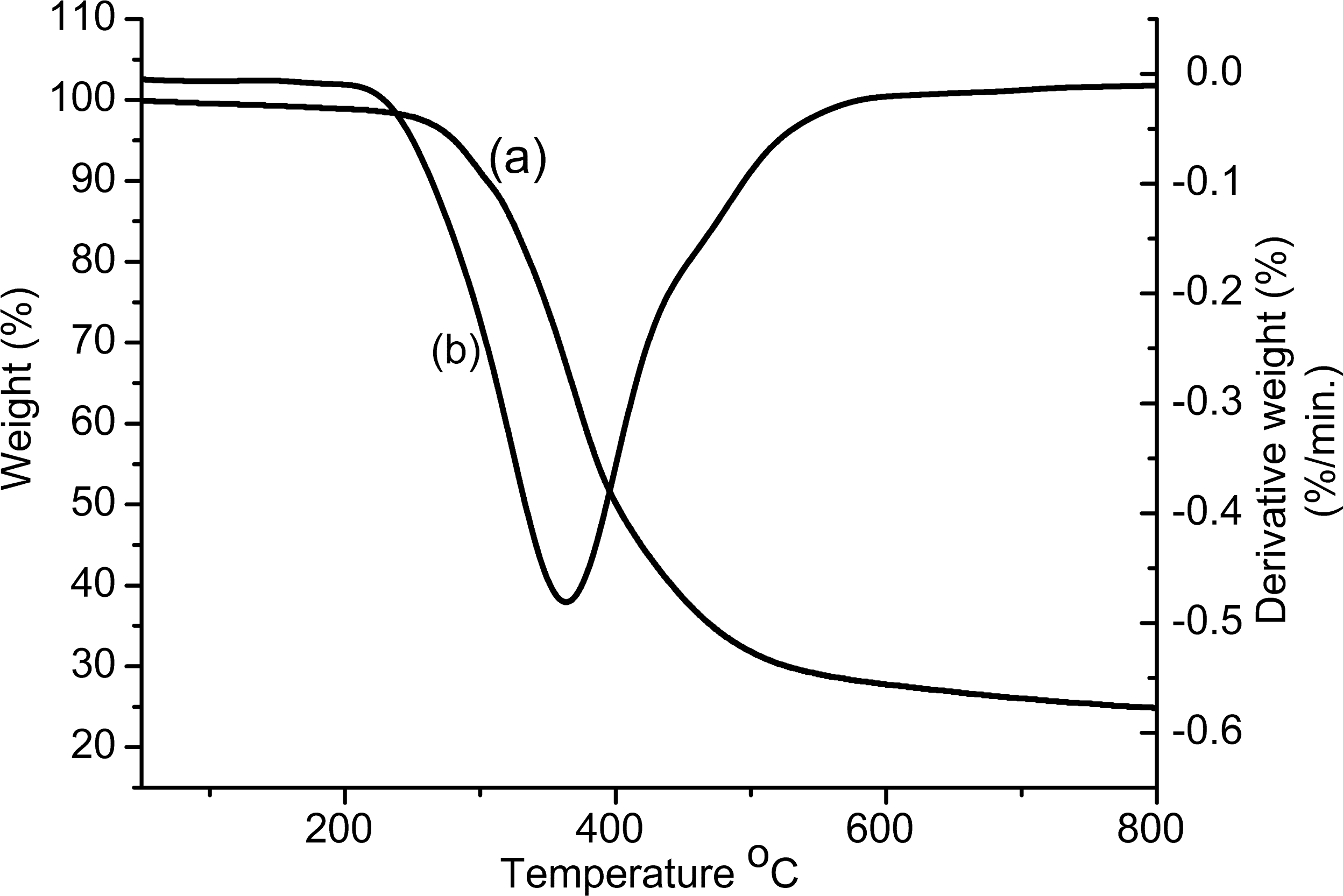
| Polymer | Co-catalyst | Yield (%) | Mnb | Mw/Mnb |
|---|---|---|---|---|
| PAT-1 | Triethylamine | 12 | 2790 | 1.46 |
| PAT-2 | Diisopropylamine | 20 | 4460 | 1.67 |
| PAT-3 | Butylamine | 6 | 4690 | 1.33 |
| Electrode | Epc/V | Epa/V | E1/2/V vs. (Ag/AgCl) | EFc/V vs.(Ag/AgCl) | E1/2/V vs. Fc | LUMO (eV) |
| Pt disc | 0.80 | −0.33 | 0.24 | 0.47 | 0.71 | 4.09 |
| −0.61 | −0.77 | 0.69 | 0.47 | 1.16 | 3.64 |
| Polymer | T5%a (°C) | T10%b (°C) | Tcd max (°C) | Ycd at 500°C (%) | Ref. |
|---|---|---|---|---|---|
| PAT-2 | 230 | 248 | 363 | 29 | This work |
| Poly(phenylacetylene) | ∼264 | ∼280 | -- | ∼12 | [35] |
Share and Cite
Koz, B.; Kiskan, B.; Yagci, Y. Synthesis and Characterization of Polyacetylene with Side-chain Thiophene Functionality. Int. J. Mol. Sci. 2008, 9, 383-393. https://doi.org/10.3390/ijms9030383
Koz B, Kiskan B, Yagci Y. Synthesis and Characterization of Polyacetylene with Side-chain Thiophene Functionality. International Journal of Molecular Sciences. 2008; 9(3):383-393. https://doi.org/10.3390/ijms9030383
Chicago/Turabian StyleKoz, Banu, Baris Kiskan, and Yusuf Yagci. 2008. "Synthesis and Characterization of Polyacetylene with Side-chain Thiophene Functionality" International Journal of Molecular Sciences 9, no. 3: 383-393. https://doi.org/10.3390/ijms9030383
APA StyleKoz, B., Kiskan, B., & Yagci, Y. (2008). Synthesis and Characterization of Polyacetylene with Side-chain Thiophene Functionality. International Journal of Molecular Sciences, 9(3), 383-393. https://doi.org/10.3390/ijms9030383





