Vasorelaxant and Antioxidant Activities of Spilanthes acmella Murr.
Abstract
:1. Introduction
2. Results
2.1. Components and HPLC (high performance liquid chromatography) profiles of the extracts
2.2. Vasorelaxant activity
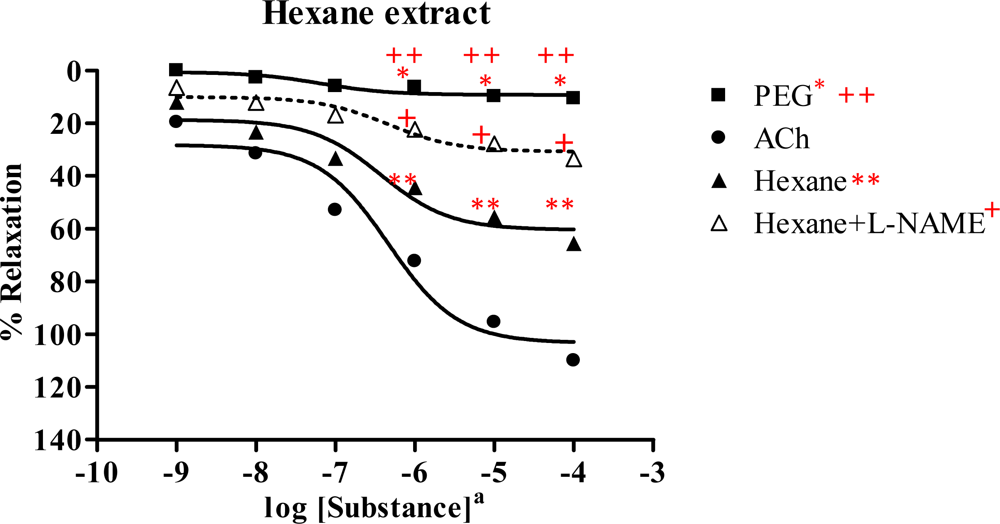
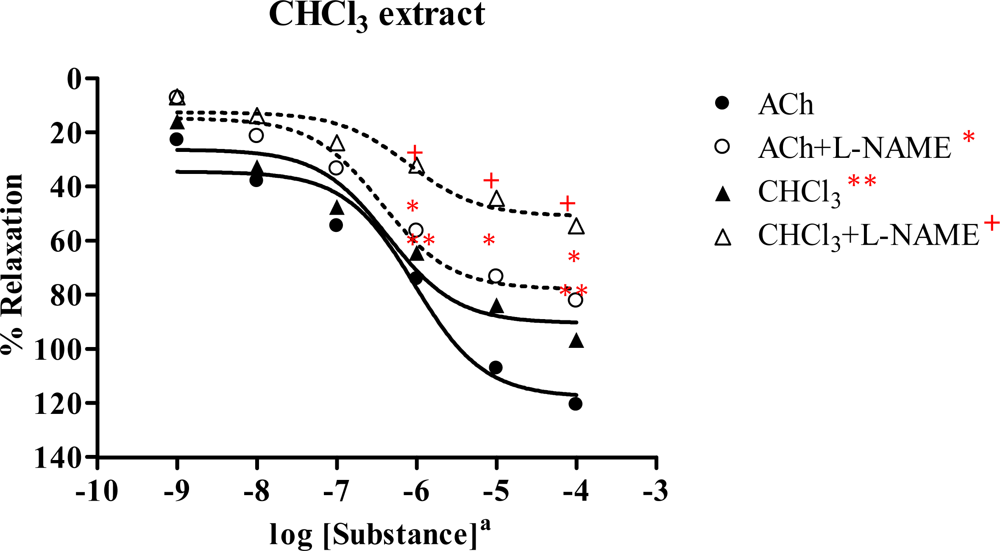
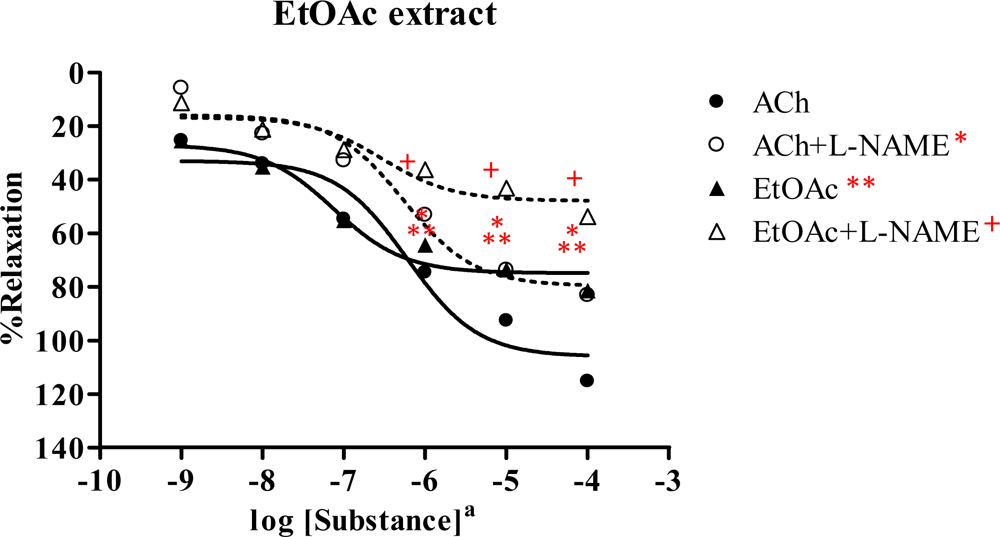
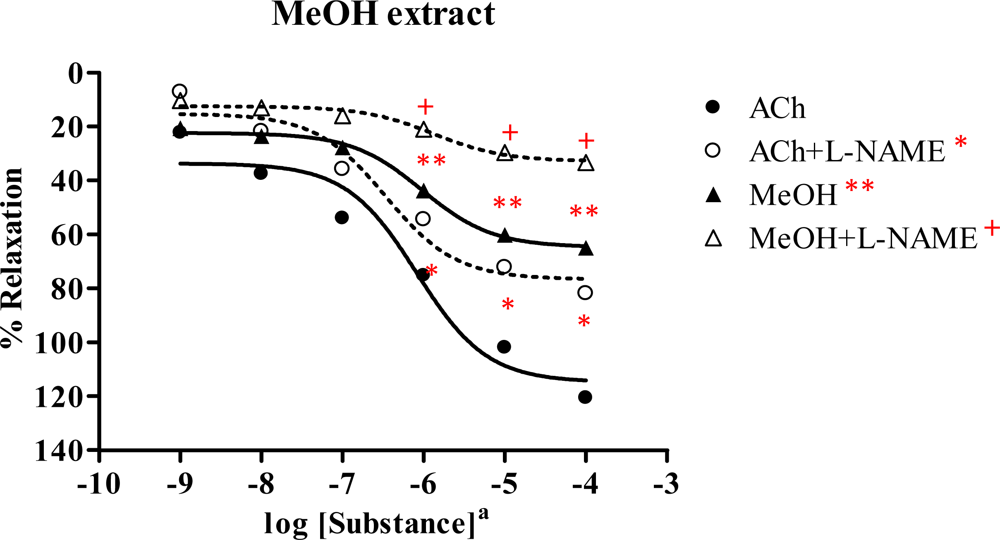
2.3. Effect of Spilanthes acmella Murr. extracts on the vascular function of rat thoracic aorta in the absence or presence of a NOS inhibitor (L-NAME, 1 mM)
2.3.1. Hexane extract
2.3.2. Chloroform extract
2.3.3. Ethyl acetate extract
2.3.4. Methanol extract
2.4. Effect of Spilanthes acmella Murr. on the vascular function of rat thoracic aorta in the absence of endothelial cells
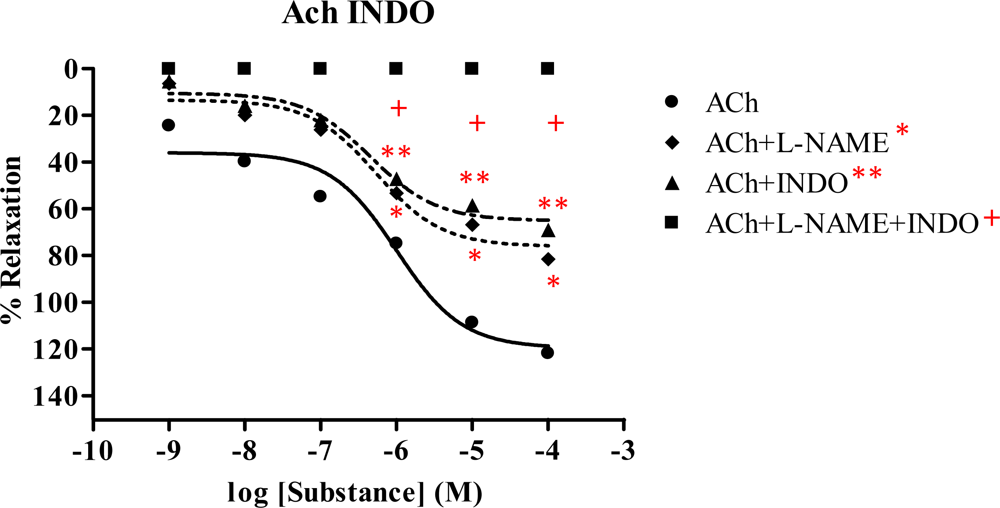
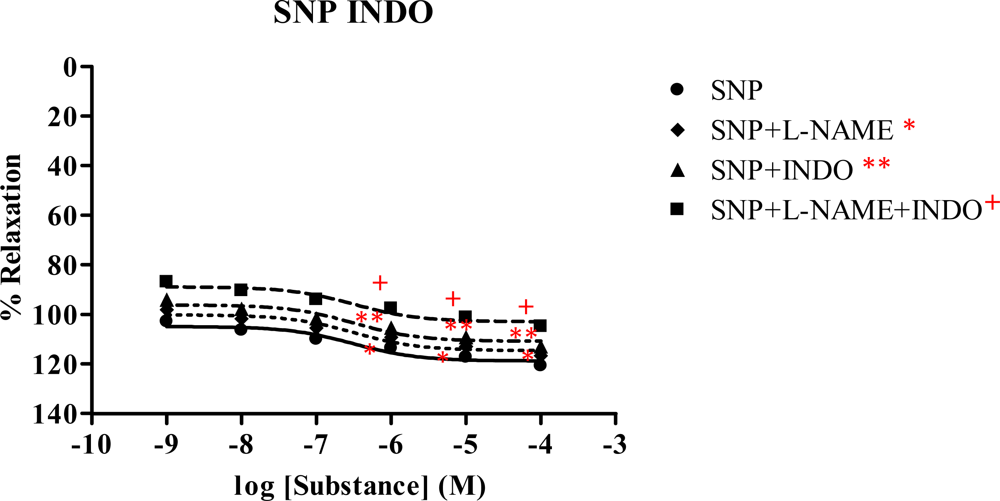
2.5. Effect of Spilanthes acmella Murr. on the vascular function of rat thoracic aorta in the presence of a cyclooxygenase inhibitor (INDO)
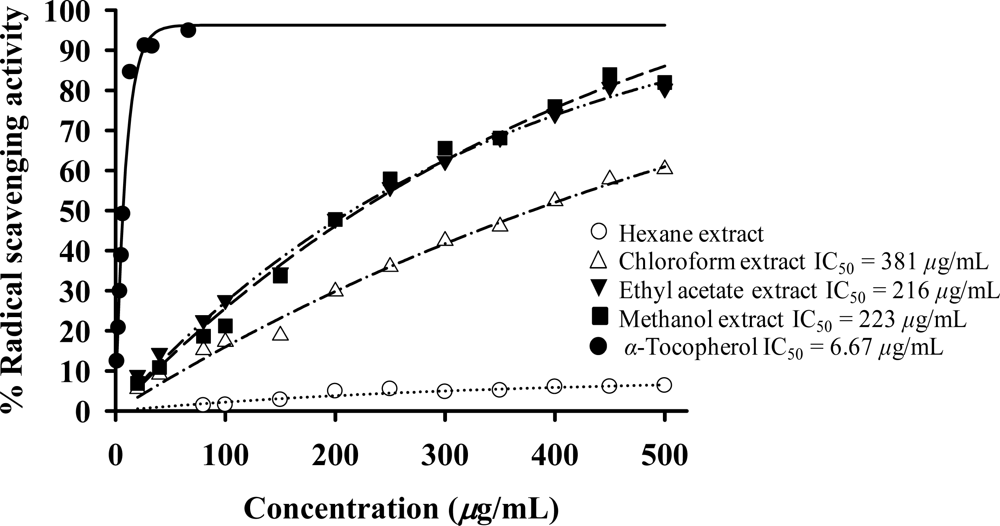
2.6. Antioxidant activity
3. Discussion
4. Experimental Section
4.1. Materials
4.1.1. Chemicals
4.1.2. Plant materials
4.2. Methods
4.2.1. Extraction
4.2.2. Components and HPLC profiles of the extracts
4.2.3. Biological activity
4.2.3.1. Vasorelaxant assay
4.2.3.1.1. Isometric tension measurements
4.2.3.1.2. Statistical analyses
4.2.3.2. Antioxidative assay
5. Conclusions
Acknowledgments
References
- Chopra, RN; Nayara, SL; Chopra, IC. Glossary of Indian medicinal plants; Council of Scientific & Industrial Research: New Delhi, 1956; pp. 168–169. [Google Scholar]
- Bunyapraphatsara, N; Chokechareunporn, O. Tradition medicinal plants; Prachachon: Bangkok, 1999. [Google Scholar]
- Farnsworth, NR; Bunyapraphatsara, N. Thai medicinal plants recommended for primary health care system; Prachachon: Bangkok, 1992. [Google Scholar]
- Perry, LM; Metzger, J. Medicinal plants of East and South-East Asia; MIT Press: Cambridge, 1980. [Google Scholar]
- Wilkinson, J. Herbs and flowers of the cottage garden; Book Builders: Melbourne, 1989. [Google Scholar]
- Gokhale, VG; Bhide, BV. Chemical investigation of Spilanthes acmella. J. Ind. Chem. Soc 1945, 22, 250–252. [Google Scholar]
- Ramsewak, RS; Erickson, AJ; Nair, MG. Bioactive N-isobutylamides from the flower buds of Spilanthes acmella. Phytochemistry 1999, 51, 729–732. [Google Scholar]
- Krishnaswamy, NR; Prasanna, S. α and β-Amyrin esters and sitosterol glucoside from Spilanthes acmella. Phytochemistry 1975, 14, 1666–1667. [Google Scholar]
- Mukharya, DK; Ansari, AH. Olean-12-en-3-O-beta-D-galactopyranosyl (1→4)-O-alpha-L-rhamnopyranoside: A new triterpenoidal saponin from the roots of Spilanthes acmella (Murr.). Indian J. Chem. B 1987, 26, 86. [Google Scholar]
- Saengsirinavin, C; Vanveerakul, B; Chalermsanyakorn, P. Local anesthetic action of Spilanthes acmella. Murr Siriraj Hospital Gazazette 1990, 42, 123–131. [Google Scholar]
- Ansari, AH; Mukharya, DK; Saxena, VK. Analgesic study of N-isobutyl-4,5-decadienamide isolated from the flowers of Spilanthes acmella (Murr). Indian J. Pharm. Sci 1988, 50, 106. [Google Scholar]
- Ratnasooriya, WD; Pieris, KPP. Attenuation of persistent pain and hyperalgesia by Spilanthus acmella flowers in rats. Pharm. Biol 2005, 43, 614–619. [Google Scholar]
- Saraf, DK; Dixit, VK. Spilanthes acmella Murr.: Study on its extract spilanthol as larvicidal compound. Asian J. Exp. Sci 2002, 16, 9–19. [Google Scholar]
- Pitasawat, B; Choochote, W; Kanjanapothi, D; Panthong, A; Jitpakdi, A; Chaithong, U. Screening for larvicidal activity of ten carminative plants. Southeast Asian J. Trop. Med. Public Health 1998, 29, 660–662. [Google Scholar]
- Ekanem, AP; Wang, M; Simon, JE; Moreno, DA. Antiobesity properties of two African plants (Afromomum meleguetta and Spilanthes acmella) by pancreatic lipase inhibition. Phytother. Res 2007, 21, 1253–1255. [Google Scholar]
- Ratnasooriya, WD; Pieris, KPP; Samaratunga, U; Jayakody, JRAC. Diuretic activity of Spilanthes acmella flowers in rats. J. Ethnopharmacol 2004, 91, 317–320. [Google Scholar]
- Pandey, HK; Rawut, PS; Kumar, N; Gauri, S. A herbal formulation for toothache and related disorders and a process for preparation thereof. IN Patent 2004DE00260. Chem. Abstr 2007, 147, 350526. [Google Scholar]
- Adler, RJ. Compositions for the acute and/or long term treatment of periodontal diseases using herb extracts. Eur Patent WO 2006059196. Chem. Abstr 2006, 145, 14791. [Google Scholar]
- Bajarang, BB. Invention of herbal drug against HIV infection/AIDS. IN Patent 2005MU01512. Chem. Abstr 2007, 147, 413250. [Google Scholar]
- Quignard, JF; Félétou, M; Thollon, C; Vilaine, JP; Duhault, J; Vanhoutte, PM. Potassium ions and endothelium-derived hyperpolarizing factor in guinea-pig carotid and porcine coronary arteries. Br. J. Phrmacol 1999, 127, 27–34. [Google Scholar]
- Ferrer, M; Marin, J; Encabo, A; Alonso, MJ; Balfagón, G. Role of K+ channels and sodium pump in the vasodilatation induced by acetylcholine, nitric oxide and cyclic GMP in the rabbit aorta. Gen. Pharmacol 1999, 33, 35–41. [Google Scholar]
- Edwards, G; Dora, KA; Gardener, MJ; Garland, CJ; Weston, AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 1998, 396, 269–272. [Google Scholar]
- Zardi, EM; Dobrina, A; Amoroso, A; Afeltra, A. Prostacyclin in liver disease: A potential therapeutic option. Expert Opin. Biol .Ther 2007, 7, 785–790. [Google Scholar]
- Piccinelli, AL; Arana, S; Caceres, A; Bianca, REV; Sorrentino, R; Rastrelli, L. New lignans from the roots of Valeriana prionophylla with antioxidative and vasorelaxant activities. J. Nat. Prod 2004, 67, 1135–1140. [Google Scholar]
- Zamblé, A; Martin-Nizard, F; Sahpaz, S; Hennebelle, T; Staels, B; Bordet, R; Duriez, P; Brunet, C; Bailleul, F. Vasoactivity, antioxidant and aphrodisiac properties of Caesalpinia benthamiana roots. J. Ethnopharmacol 2008, 116, 112–119. [Google Scholar]
- Rodríguez-Rodríguez, R; Herrera, MD; Perona, JS; Ruiz-Gutiérrez, V. Potential vasorelaxant effects of oleanolic acid and erythrodiol, two triterpenoids contained in ‘orujo’ olive oil, on rat aorta. Br. J. Nutr 2004, 92, 635–642. [Google Scholar]
- Demirci, B; McKeown, PP; Bayraktutan, DVMU. The bimodal regulation of vascular function by superoxide anion: Role of endothelium. BMB rep 2008, 41, 223–229. [Google Scholar]
- Shin, JI; Lee, YK; Kim, YM; Hwang, JT; Park, OJ. Possible link between NO concentrations and COX-2 expression in systems treated with soy-isoflavones. Ann. N. Y. Acad. Sci 2007, 1095, 564–573. [Google Scholar]
- Zenebe, W; Pechánová, O; Andriantsitohaina, R. Red wine polyphenols induce vasorelaxation by increased nitric oxide bioactivity. Physiol. Res 2003, 52, 425–432. [Google Scholar]
- Villa, IC; Vera, R; Galisteo, M; O’Valle, F; Romero, M; Zarzuelo, A; Duarte, J. Endothelial nitric oxide production stimulated by bioflavonoid chrysin in rat isolated arota. Planta. Med 2005, 71, 829–834. [Google Scholar]
- Norton, C; Kalea, AZ; Harris, PD; Klimis-Zacas, DJ. Wild blueberry-rich diets affect the contractile machinery of the vascular smooth muscle in the Sprague-Dawley rat. J. Med. Food 2005, 8, 8–13. [Google Scholar]
- DalBó, S; Moreira, EG; Brandão, FC; Horst, H; Pizzolatti, MG; Micke, GA; Ribeiro-do-Valle, RM. Mechanisms underlying the vasorelaxant effect induced by proanthocyanidin-rich fraction from Croton celtidifolius in rat small resistance arteries. J. Pharmacol. Sci 2008, 106, 234–241. [Google Scholar]
- Bate-Smith, EC; Swain, T. The isolation of 2,4,4′-trihydroxychalcone from yellow varieties of Dahlia variabilis. J. Chem. Soc 1953, 2185–2187. [Google Scholar]
- Prachayasittikul, S; Buraparuangsang, P; Worachartcheewan, A; Isarankura-Na-Ayudhya, C; Ruchirawat, S; Prachayasittikul, V. Antimicrobial and antioxidative activities of bioactive constituents from Hydnophytum formicarum Jack. Molecules 2008, 13, 904–921. [Google Scholar]
- Baisch, ALM; Urban, H; Ruiz, AN. Endothelium-dependent vasorelaxing activity of aqueous extracts of lyophilized seeds of Casimiroa edulis (AECe) on rat mesenteric arterial bed. J. Ethnopharmacol 2004, 95, 163–167. [Google Scholar]
- Dongmo, AB; Azebaze, AGB; Nguelefack, TB; Ouahouo, BM; Sontia, B; Meyer, M; Nkengfack, AE; Kamanyi, A; Vierling, W. Vasodilator effect of the extracts and some coumarins from the stem bark of Mammea africana (Guttiferae). J. Ethnopharmacol 2007, 111, 329–334. [Google Scholar]
- Jiang, HD; Cai, J; Xu, J-H; Zhou, X-M; Xia, Q. Endothelium-dependent and direct relaxation induced by ethyl acetate extract from Flos Chrysanthemi in rat thoracic aorta. J. Ethnopharmacol 2005, 101, 221–226. [Google Scholar]
- Yin, HH; Kang, DG; Choi, DH; Kwon, TO; Lee, HS. Screening of vasorelaxant activity of some medicinal plants used in Oriental medicines. J. Ethnopharmacol 2005, 99, 113–117. [Google Scholar]
- Magos, GA; Mateos, JC; Páez, E; Fernández, G; Lobato, C; Márquez, C; Enríquez, RG. Hypotensive and vasorelaxant effects of the procyanidin fraction from Guazuma ulmifolia bark in normotensive and hypertensive rats. J. Ethnopharmacol 2008, 117, 58–68. [Google Scholar]
- Siemonsma, JS; Piluck, K. Plant resources of South East Asia No 8 Vegetable; Pudoc Scientific Publishers: Wageningen, The Netherlands, 1993. [Google Scholar]
- Woodman, OL; Wongsawatkul, O; Sobey, CG. Contribution of nitric oxide, cyclic GMP and K+ channel to acetylcholine induced dilation of rat conduit and resistance arteries. Clin. Exp. Pharmacol. Toxicol 2000, 27, 32–34. [Google Scholar]
- Prachayasittikul, S; Suksrichavalit, T; Isarankura-Na-Ayudhya, C; Ruchirawat, S; Prachayasittikul, V. Antimicrobial and antioxidative activities of 1-Adamantylthio derivatives of 3-substitued pyridines. EXCLI J 2008, 7, 63–70. [Google Scholar]
- Piacham, T; Isarankura-Na-Ayudhya, C; Nantasenamat, C; Yainoy, S; Ye, L; Prachayasittikul, V. Metalloantibiotic Mn(II)-bacitracin complex mimicking manganese superoxide dismutase. Biochem. Biophys. Res. Comm 2006, 341, 925–930. [Google Scholar]




| Extract | RT (min) | Area (%) | Extract | RT (min) | Area (%) |
|---|---|---|---|---|---|
| Hexane | 48.80 | 3.86 | Chloroformb | 25.65 | 0.61 |
| 49.58 | 4.20 | 27.20 | 0.37 | ||
| 50.42 | 7.79 | 27.81 | 0.55 | ||
| 51.64 | 5.38 | 30.95 | 1.69 | ||
| 52.56 | 66.06 | 32.14 | 4.72 | ||
| 56.59 | 5.09 | ||||
| 57.76 | 0.97 | Ethyl acetateb | 18.27 | 2.55 | |
| 58.38 | 6.64 | 19.92 | 0.84 | ||
| 22.89 | 1.57 | ||||
| Chloroforma | 52.54 | 65.72 | 30.95 | 0.72 | |
| 32.17 | 0.22 | ||||
| Ethyl acetatea | 52.58 | 67.34 | 33.73 | 0.38 | |
| Methanola | 52.60 | 64.18 | Methanolb | 23.82 | 2.64 |
| Compound | Vasorelaxant activity | |||
|---|---|---|---|---|
| Without l-NAME | With l-NAME (1 mM) | |||
| Rmax (%) | ED50 (mg/mL) | Rmax (%) | ED50 (mg/mL) | |
| Hexane extracta | 65.67 ± 0.984 | 3.601×10−7 | 33.49 ± 1.122 | 4.805×10−7 |
| ACha | 110.08 ± 1.801 | 4.657×10−7 | NA | NA |
| Chloroform extractb | 96.64 ± 1.112 | 4.279×10−7 | 54.38 ± 0.575 | 1.003×10−6 |
| ACha | 120.63 ± 1.671 | 9.428×10−7 | 82.15 ± 1.158 | 3.611×10−7 |
| Ethyl acetate extractb | 81.64 ± 0.530 | 7.614×10−8 | 53.99 ± 1.517 | 3.885×10−7 |
| AChb | 115.47 ± 0.951 | 5.912×10−7 | 83.36 ± 1.169 | 5.650×10−7 |
| Methanol extracta | 65.09 ± 0.409 | 9.550×10−7 | 33.38 ± 0.403 | 1.375×10−6 |
| ACha | 120.69 ± 0.220 | 8.183×10−7 | 82.01 ± 0.582 | 3.427×10−7 |
| AChb | 119.20 ± 0.344 | 7.878×10−7 | 80.86 ± 0.368 | 4.163×10−7 |
| SNPb | 120.53 ± 2.270 | 1.727×10−7 | 114.84 ± 0.716 | 3.171×10−7 |
| Compounda | Vasorelaxant activity | |||
|---|---|---|---|---|
| +Et | −Et | |||
| Rmax (%) | ED50 (mg/mL) | Rmax (%) | ED50 (mg/mL) | |
| Hexane extract | 64.82 ± 0.870 | 2.556×10−7 | 0 | - |
| ACh | 104.31 ± 1.595 | 2.440×10−7 | 7.16 ± 2.010 | 3.128×10−7 |
| Chloroform extract | 98.00 ± 0.694 | 4.422×10−7 | 5.64 ± 0.080 | 3.128×10−7 |
| ACh | 120.36 ± 0.823 | 7.534×10−7 | 7.42 ± 0.159 | 3.128×10−7 |
| Ethyl acetate extract | 81.68 ± 0.682 | 7.638×10−8 | 0 | - |
| ACh | 118.58 ± 0.550 | 7.477×10−7 | 7.42 ± 0.159 | 3.128×10−7 |
| Methanol extract | 65.62 ± 0.651 | 1.002×10−6 | 0 | - |
| ACh | 119.93 ± 0.495 | 7.967×10−7 | 7.28 ± 0.138 | 3.128×10−7 |
| Compound | Vasorelaxant activity | |||||||
|---|---|---|---|---|---|---|---|---|
| −Inhibitor | +l-NAME (1 mM) | +INDO (1 mM) | +l-NAME (1 mM) + INDO (1 mM) | |||||
| Rmax (%) | ED50 (mg/mL) | Rmax (%) | ED50 (mg/mL) | Rmax(%) | ED50 (mg/mL) | Rmax (%) | ED50 (mg/mL) | |
| ACha | 121.74 ± 1.440 | 9.990×10−7 | 81.34 ± 0.770 | 5.455×10−7 | 68.78 ± 0.919 | 4.575×10−7 | 0 | - |
| Hexane extracta | 69.05 ± 0.693 | 5.016×10−7 | 31.29 ± 0.619 | 3.163×10−7 | 26.64 ± 0.768 | 3.162×10−7 | 0 | - |
| Chloroform extracta | 94.25 ± 0.873 | 5.278×10−7 | 51.62 ± 0.706 | 1.598×10−6 | 46.70 ± 0.511 | 6.661×10−7 | 0 | - |
| Ethyl acetate extracta | 85.84 ± 1.196 | 8.552×10−8 | 52.95 ± 0.976 | 7.804×10−7 | 44.48 ± 0.350 | 3.133×10−7 | 0 | - |
| Methanol extractb | 65.56 ± 0.535 | 9.444×10−7 | 33.24 ± 0.608 | 1.404×10−6 | 32.19 ± 0.420 | 1.072×10−6 | 0 | - |
| SNPb | 120.84 ± 1.176 | 3.164×10−7 | 116.70 ± 1.290 | 3.167×10−7 | 112.93 ± 0.613 | 3.155×10−7 | 104.98 ± 1.407 | 3.165×10−7 |
| Extract | % Radical scavenging activitya (DPPH assay); 200 μg/mL | % NBT inhibitionb (SOD assay); 200 μg/mL |
|---|---|---|
| Hexane | 4.90 | 0.41 |
| Chloroform | 29.82 | 57.92 |
| Ethyl acetate | 47.90 | 33.05 |
| Methanol | 47.76 | 47.02 |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/). This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wongsawatkul, O.; Prachayasittikul, S.; Isarankura-Na-Ayudhya, C.; Satayavivad, J.; Ruchirawat, S.; Prachayasittikul, V. Vasorelaxant and Antioxidant Activities of Spilanthes acmella Murr. Int. J. Mol. Sci. 2008, 9, 2724-2744. https://doi.org/10.3390/ijms9122724
Wongsawatkul O, Prachayasittikul S, Isarankura-Na-Ayudhya C, Satayavivad J, Ruchirawat S, Prachayasittikul V. Vasorelaxant and Antioxidant Activities of Spilanthes acmella Murr. International Journal of Molecular Sciences. 2008; 9(12):2724-2744. https://doi.org/10.3390/ijms9122724
Chicago/Turabian StyleWongsawatkul, Orapin, Supaluk Prachayasittikul, Chartchalerm Isarankura-Na-Ayudhya, Jutamaad Satayavivad, Somsak Ruchirawat, and Virapong Prachayasittikul. 2008. "Vasorelaxant and Antioxidant Activities of Spilanthes acmella Murr." International Journal of Molecular Sciences 9, no. 12: 2724-2744. https://doi.org/10.3390/ijms9122724
APA StyleWongsawatkul, O., Prachayasittikul, S., Isarankura-Na-Ayudhya, C., Satayavivad, J., Ruchirawat, S., & Prachayasittikul, V. (2008). Vasorelaxant and Antioxidant Activities of Spilanthes acmella Murr. International Journal of Molecular Sciences, 9(12), 2724-2744. https://doi.org/10.3390/ijms9122724




