Germacrene D Cyclization: An Ab Initio Investigation
Abstract
:1. Introduction
2. Results and Discussion
3. Computational Methods
References and Notes
- Setzer, WN; Haber, WA. Leaf essential oil composition of five species of Beilschmiedia from Monteverde, Costa Rica. Nat Prod Commun 2007, 2, 79–83. [Google Scholar]
- Eason, HM; Setzer, WN. Bark essential oil composition of Cedrela tonduzii C. DC. (Meliaceae) from Monteverde, Costa Rica. Rec Nat Prod 2007, 1, 24–27. [Google Scholar]
- Setzer, WN. Chemical compositions of the bark essential oils of Croton monteverdensis and Croton niveus from Monteverde, Costa Rica. Nat Prod Commun 2006, 1, 567–572. [Google Scholar]
- Cole, RA; Haber, WA; Setzer, WN. Chemical composition of essential oils of seven species of Eugenia from Monteverde, Costa Rica. Biochem Syst Ecol 2007, 35, 877–886. [Google Scholar]
- Takaku, S; Haber, WA; Setzer, WN. Leaf essential oil composition of 10 species of Ocotea (Lauraceae) from Monteverde, Costa Rica. Biochem Syst Ecol 2007, 35, 525–532. [Google Scholar]
- Setzer, WN; Park, G; Agius, BR; Stokes, SL; Walker, TM; Haber, WA. Chemical compositions and biological activities of leaf essential oils of twelve species of Piper from Monteverde, Costa Rica. Molecules 2008, 13. (submitted). [Google Scholar]
- Yoshihara, K; Ohta, Y; Sakai, T; Hirose, Y. Germacrene D, a key intermediate of cadinene group compounds and bourbonenes. Tetrahedron Lett 1969, 2263–2264. [Google Scholar]
- Bülow, N; König, WA. The role of germacrene D as a precursor in sesquiterpene biosynthesis: investigations of acid catalyzed, photochemically and thermally induced rearrangements. Phytochemistry 2000, 55, 141–168. [Google Scholar]
- Bartley, JP; Foley, P. Supercritical fluid extraction of Australian-grown ginger (Zingiber officinale). J Sci Food Agric 1994, 66, 365–371. [Google Scholar]
- Asfaw, N; Storesund, HJ; Skattebøl, L; Aasen, AJ. Coexistence of chrysanthenone, filifolone, and (Z)-isogeranic acid in hydrodistillates. Artefacts! Phytochemistry 2001, 58, 489–492. [Google Scholar]
- Babu, KGD; Kaul, VK. Variation in essential oil composition of rose-scented geranium (Pelargonium sp.) distilled by different distillation techniques. Flavour Fragr J 2005, 20, 222–231. [Google Scholar]
- Teixeira, S; Mendes, A; Alves, A; Santos, L. Simultaneous distillation-extraction of high-value volatile compounds from Cistus ladanifer L. Anal Chim Acta 2007, 584, 439–446. [Google Scholar]
- Palá-Paúl, J; Pérez-Alonso, MJ; Velasco-Negueruela, A; Vadaré, J; Villa, AM; Sanz, J; Brophy, JJ. Essential oil composition of the different parts of Eryngium bourgatii Gouan from Spain. J Chromatogr A 2005, 1074, 235–239. [Google Scholar]
- Roussis, V; Tsoukatou, M; Petrakis, PV; Chinou, I; Skoula, M; Harborne, JB. Volatile constituents of four Helichrysum species growing in Greece. Biochem Syst Ecol 2000, 28, 163–175. [Google Scholar]
- Juteau, F; Masotti, V; Bessière, JM; Viano, J. Compositional characteristics of the essential oil of Artemisia campestris var glutinosa. Biochem Syst Ecol 2002, 30, 1065–1070. [Google Scholar]
- Maia, JGS; Zoghbi, MdGB; Andrade, EHA; da Silva, MH; Luz, AIR; da Silva, JD. Essential oils composition of Eupatorium species growing wild in the Amazon. Biochem Syst Ecol 2002, 30, 1071–1077. [Google Scholar]
- Chericoni, S; Flamini, G; Campeol, E; Cioni, PL; Morelli, I. GC-MS analyses of the essential oil from the aerial parts of Artemisia verlotiorum: variability during the year. Biochem Syst Ecol 2004, 32, 423–429. [Google Scholar]
- Gauvin, A; Smadja, J. Essential oil composition of four Psiadia species from Reunion Island: A chemotaxonomic study. Biochem Syst Ecol 2005, 33, 705–714. [Google Scholar]
- Saroglou, V; Dorizas, N; Kypriotakis, Z; Skaltsa, H. Analysis of the essential oil composition of eight Anthemis species from Greece. J Chromatogr A 2006, 1104, 313–322. [Google Scholar]
- Demetzos, C; Angelopoulou, D; Perdetzoglou, D. A comparative study of the essential oils of Cistus salviifolius in several populations of Crete (Greece). Biochem Syst Ecol 2002, 30, 651–665. [Google Scholar]
- Nogueira, PCdL; Bittrich, V; Shepherd, GJ; Lopes, AV; Marsaioli, AJ. The ecological and taxonomic importance of flower volatiles of Clusia species (Guttiferae). Phytochemistry 2001, 56, 443–452. [Google Scholar]
- Schwob, I; Bessière, JM; Viano, J. Composition of the essential oils of Hypericum perforatum L. from southeastern France. C R Biol 2002, 325, 781–785. [Google Scholar]
- Schwob, I; Bessiere, JM; Masotti, V; Viano, J. Changes in essential oil composition in Saint John's wort (Hypericum perforatum L.) aerial parts during its phenological cycle. Biochem Syst Ecol 2004, 32, 735–745. [Google Scholar]
- Petrakis, PV; Couladis, M; Roussis, V. A method for detecting the biosystematic significance of the essential oil composition: The case of five Hellenic Hypericum L. species. Biochem Syst Ecol 2005, 33, 873–898. [Google Scholar]
- Saroglou, V; Marin, PD; Rancic, A; Veljic, M; Skaltsa, H. Composition and antimicrobial activity of the essential oil of six Hypericum species from Serbia. Biochem Syst Ecol 2007, 35, 146–152. [Google Scholar]
- Adams, RP. Systematics of the one seeded Juniperus of the eastern hemisphere based on leaf essential oils and random amplified polymorphic DNAs (RAPDs). Biochem Syst Ecol 2000, 28, 529–543. [Google Scholar]
- Salido, S; Altarejos, J; Nogueras, M; Sánchez, A; Pannecouque, C; Witvrouw, M; De Clercq, E. Chemical studies of essential oils of Juniperus oxycedrus ssp. badia. J Ethnopharmacol 2002, 81, 129–134. [Google Scholar]
- Cavaleiro, C; Salgueiro, LR; da Cunha, AP; Figueiredo, AC; Barroso, JG; Bighelli, A; Casanova, J. Composition and variability of the essential oils of the leaves and berries from Juniperus navicularis. Biochem Syst Ecol 2003, 31, 193–201. [Google Scholar]
- Vichi, S; Riu-Aumatell, M; Mora-Pons, M; Guadayol, JM; Buxaderas, S; López-Tamames, E. HS-SPME coupled to GC/MS for quality control of Juniperus communis L. berries used for gin aromatization. Food Chem 2007, 105, 1748–1754. [Google Scholar]
- Sibanda, S; Chigwada, G; Poole, M; Gwebu, ET; Noletto, JA; Schmidt, JM; Rea, AI; Setzer, WN. Composition and bioactivity of the leaf essential oil of Heteropyxis dehniae from Zimbabwe. J Ethnopharmacol 2004, 92, 107–111. [Google Scholar]
- Van Vuuren, SF; Viljoen, AM; Özek, T; Demirci, B; Başer, KHC. Seasonal and geographic variation of Heteropyxis natalensis essential oil and the effect thereof on the antimicrobial activity. S Afr J Bot 2007, 73, 441–448. [Google Scholar]
- Pereira, SI; Santos, PAG; Barroso, JG; Figueiredo, AC; Pedro, LG; Salgueiro, LR; Deans, SG; Scheffer, JJC. Chemical polymorphism of the essential oils from populations of Thymus caespititius grown on the island S. Jorge (Azores). Phytochemistry 2000, 55, 241–246. [Google Scholar]
- Skaltsa, HD; Mavrommati, A; Constantinidis, T. A chemotaxonomic investigation of volatile constituents in Stachys subsect. Swainsonianeae (Labiatae). Phytochemistry 2001, 57, 235–244. [Google Scholar]
- Veličković, DT; Randjelović, NV; Ristić, MS; Šmelcerović, AA; Veličkoviú, AS. Chemical composition and antimicrobial action of the ethanol extracts of Salvia pratensis L., Salvia glutinosa L. and Salvia aethiopis L. J Serb Chem Soc 2002, 67, 639–646. [Google Scholar]
- Couladis, M; Chinou, IB; Tzakou, O; Loukis, A. Composition and antimicrobial activity of the essential oil of Ballota pseudodictamnus L. Bentham. Phytother Res 2002, 16, 723–726. [Google Scholar]
- Ložiene, K; Vaièiuniene, J; Venskutonis, PR. Chemical composition of the essential oil of different varieties of thyme (Thymus pulegioides) growing wild in Lithuania. Biochem Syst Ecol 2003, 31, 249–259. [Google Scholar]
- Skaltsa, HD; Demetzos, C; Lazari, D; Sokovic, M. Essential oil analysis and antimicrobial activity of eight Stachys species from Greece. Phytochemistry 2003, 64, 743–752. [Google Scholar]
- Telascrea, M; de Araújo, CC; Marques, MOM; Facanali, R; de Moraes, PLR; Cavalheiro, AJ. Essential oil from the leaves of Cryptocarya mandioccana Meisner (Lauraceae): Composition and intraspecific chemical variability. Biochem Syst Ecol 2007, 35, 222–232. [Google Scholar]
- Setzer, WN; Stokes, SL; Penton, AF; Takaku, S; Haber, WA; Hansell, E; Caffrey, CR; McKerrow, JH. Cruzain inhibitory activity of leaf essential oils of Neotropical Lauraceae and essential oil components. Nat Prod Commun 2007, 2, 1203–1210. [Google Scholar]
- Agius, BR; Setzer, MC; Stokes, SL; Walker, TM; Haber, WA; Setzer, WN. Composition and bioactivity of essential oils of Lauraceae from Monteverde, Costa Rica. Int J Essent Oil Ther 2008, (in press). [Google Scholar]
- Cole, RA; Haber, WA; Lawton, RO; Setzer, WN. Leaf essential oil composition of three species of Myrcianthes from Monteverde, Costa Rica. Chem Biodiv 2008, (in press). [Google Scholar]
- Nicolić, B; Ristić, M; Bojoviæ, S; Marin, PD. Variability of the needle essential oils of Pinus heldreichii from different populations in Montenegro and Serbia. Chem Biodiv 2007, 4, 905–916. [Google Scholar]
- Fringuelli, F; Pizzo, F; Taticchi, A; Ferreira, VF; Michelotti, EL; Porter, B; Wenkert, E. Diels-Alder reactions of cycloalkenones. 4. Short syntheses of some cadinenes. J Org Chem 1985, 50, 890–891. [Google Scholar]
- Vlahov, R; Holub, M; Herout, V. On terpenes. CLXXXIV. Sesquiterpenic hydrocarbons from the essential oil of Mentha piperita of Bulgarian origin. Coll Czech Chem Comm 1967, 32, 822–829. [Google Scholar]
- Connell, DW; Hildebrand, RP; Sutherland, MD. Terpenoid chemistry XIV: the significance of the term “δ-cadinene”. Tetrahedron Lett 1968, 519–523. [Google Scholar]
- Nagasampagi, BA; Yankov, L; Dev, S. Sesquiterpenoids from the wood of Cedrela toona Roxb – partial synthesis of T-muurolol, T-cadinol and cubenol, structures of δ-cadinene and δ-cadinol. Tetrahedron Lett 1968, 1913–1918. [Google Scholar]
- Fenical, W; Sims, JJ; Wing, RM; Radlick, PC. Zonarene, a sesquiterpene from the brown seaweed Dictyopteris zonarioides. Phytochemistry 1972, 11, 1161–1163. [Google Scholar]
- Maia, BHLNS; de Paula, JR; Sant'Ana, J; da Silva, MFdGF; Fernandes, JB; Vieira, PC; Costa, MdSS; Ohashi, OS; Silva, JNM. Essential oils of Toona and Cedrela species (Meliaceae): Taxonomic and ecological implications. J Braz Chem Soc 2000, 11, 629–639. [Google Scholar]
- Mahmood, U; Kaul, VK; Singh, B. Sesquiterpene and long chain ester from Tanacetum longifolium. Phytochemistry 2002, 61, 913–917. [Google Scholar]
- El-Shazly, AM; Hussein, KT. Chemical analysis and biological activities of the essential oil of Teucrium leucocladum Boiss. (Lamiaceae). Biochem Syst Ecol 2004, 32, 665–674. [Google Scholar]
- SPARTAN ′06 for Windows; Wavefunction, Inc: Irvine, California, 2006.
- Becke, AD. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 1993, 98, 5648–5652. [Google Scholar]
- Lee, C; Yang, W; Parr, RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 1988, 37, 785–789. [Google Scholar]
- Hehre, WJ; Radom, L; Schleyer, PvR. Ab initio Molecular Orbital Theory; Wiley: New York, 1986. [Google Scholar]
| Compound | Relative Energies (kcal/mol) | Compound | Relative Energies (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B3LYP/6-31G* | MP2/6-31G** | B3LYP/6-31G* | MP2/6-31G** | ||||||
| H(0K) | G° | H(0K) | G | H(0K) | G° | H(0K) | G | ||
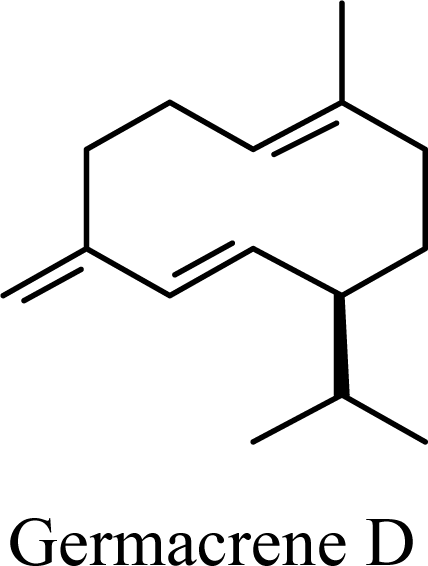 | 33.86 | 33.18 | 41.96 | 41.05 | 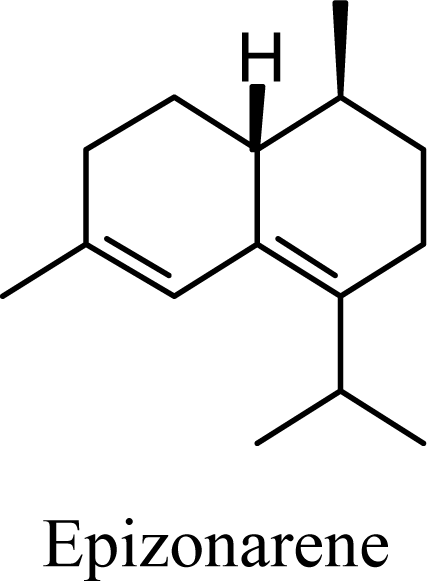 | 2.09 | 1.84 | 3.74 | 3.51 |
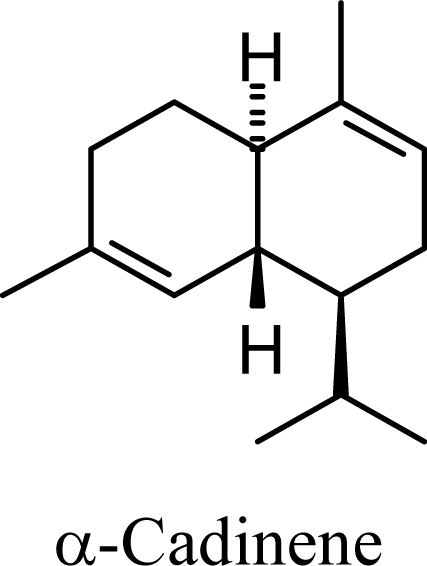 | 8.62 | 8.86 | 10.21 | 10.17 | 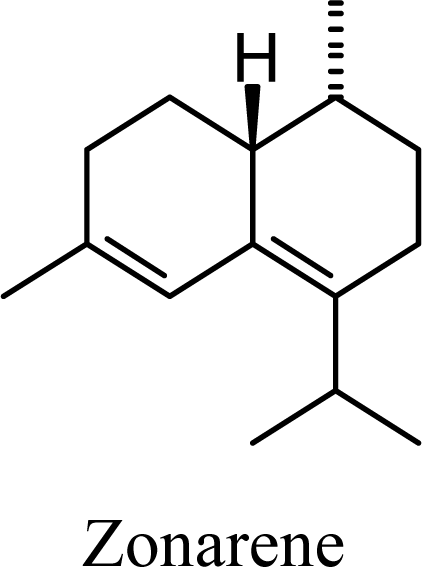 | 2.49 | 2.57 | 4.13 | 3.96 |
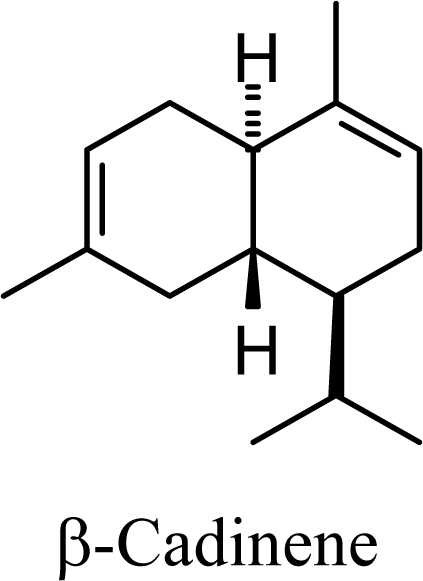 | 6.74 | 7.17 | 8.06 | 8.05 | 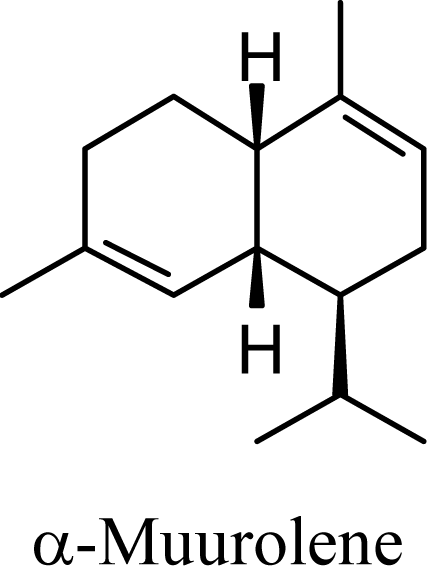 | 6.32 | 6.28 | 7.86 | 7.78 |
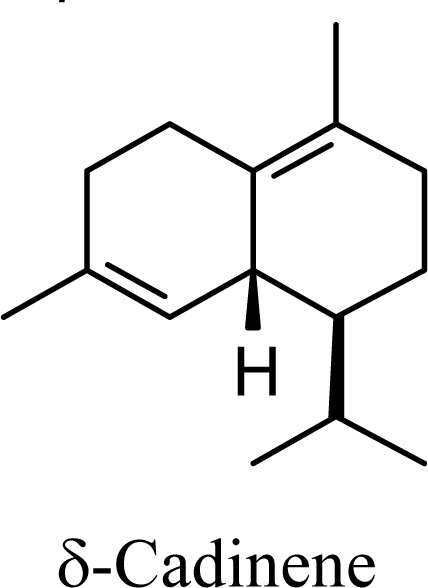 | 5.72 | 5.58 | 7.92 | 7.69 | 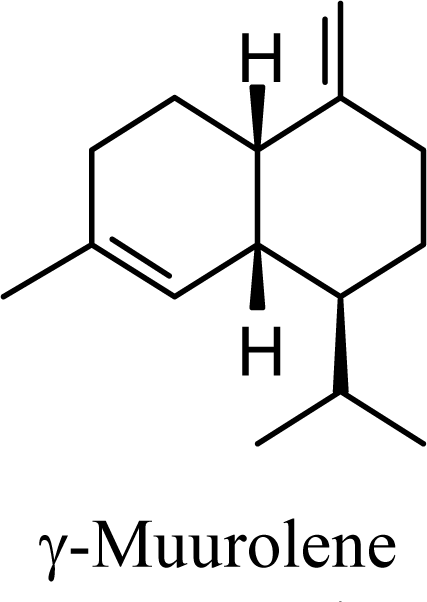 | 10.53 | 10.78 | 11.05 | 11.13 |
 | 10.68 | 11.00 | 11.79 | 11.91 | 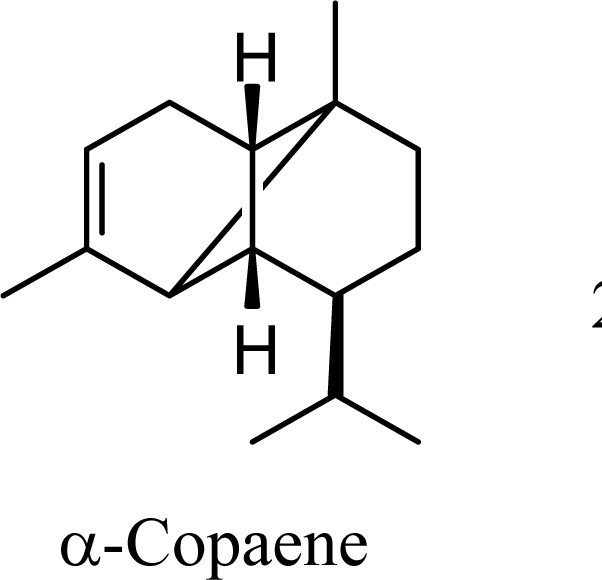 | 20.45 | 21.49 | 13.42 | 14.33 |
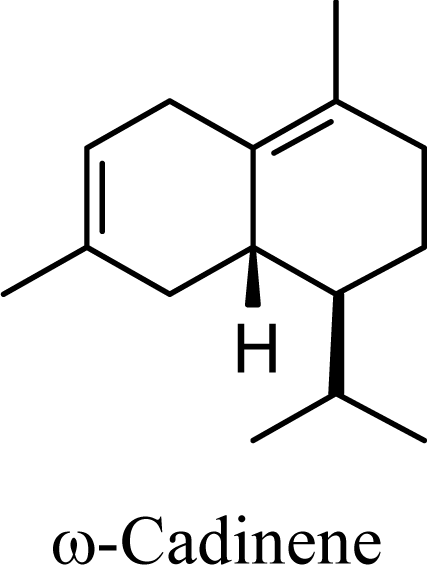 | 5.72 | 5.37 | 8.64 | 8.27 | 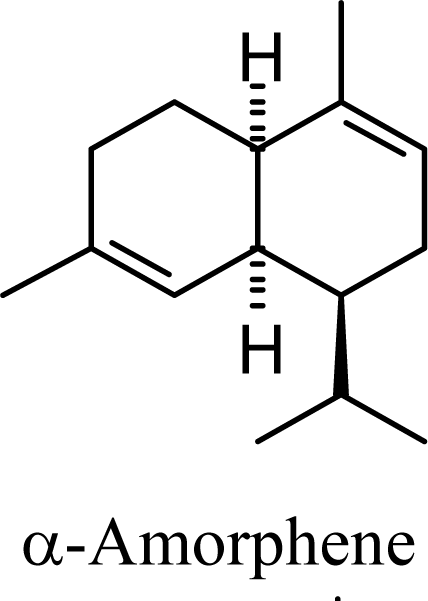 | 12.67 | 12.43 | 13.56 | 13.39 |
 | 5.58 | 5.90 | 7.52 | 7.48 | 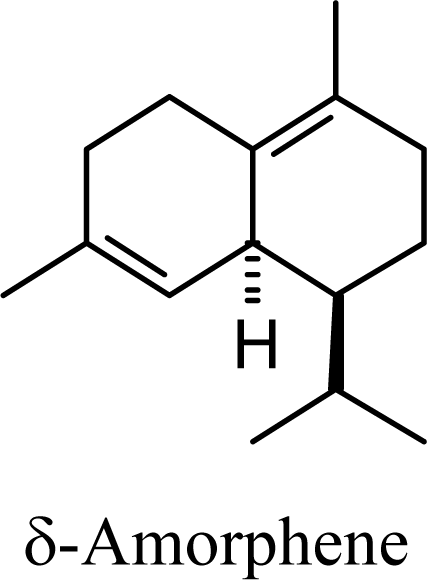 | 8.04 | 8.08 | 9.44 | 9.34 |
 | 20.02 | 20.03 | 20.61 | 20.73 | 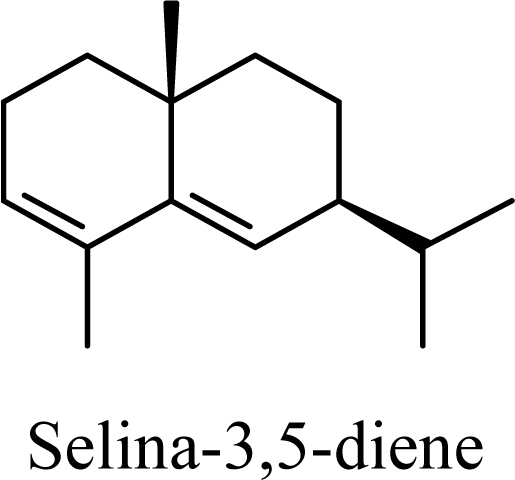 | 2.79 | 3.34 | 2.04 | 2.32 |
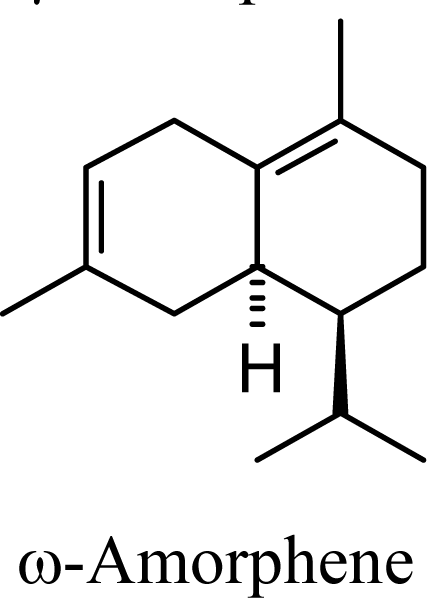 | 6.41 | 6.46 | 8.19 | 8.06 | 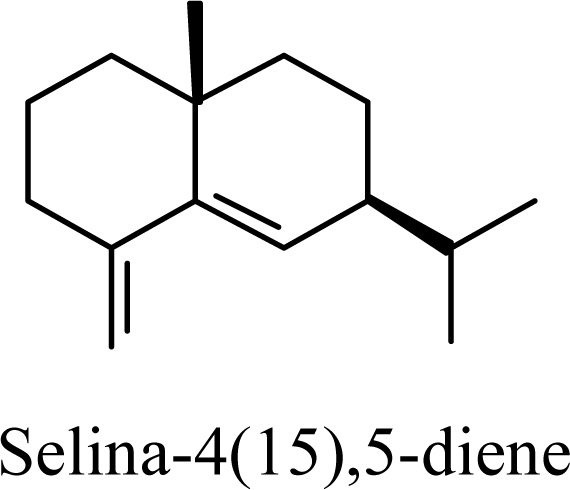 | 8.84 | 9.46 | 7.13 | 7.45 |
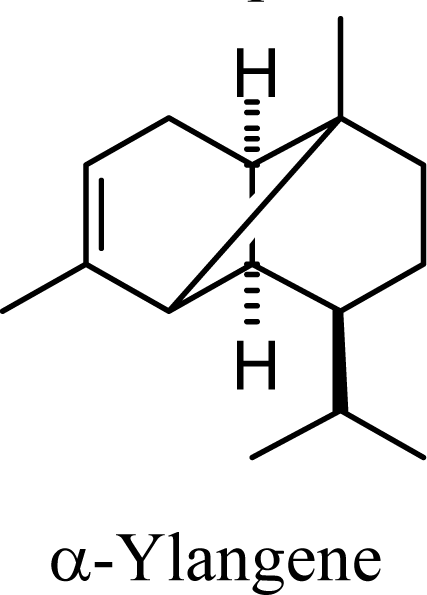 | 20.55 | 21.82 | 13.17 | 14.08 | 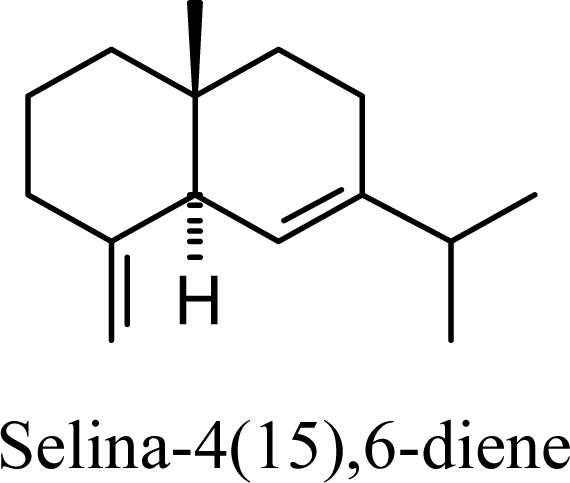 | 16.91 | 17.23 | 15.71 | 15.98 |
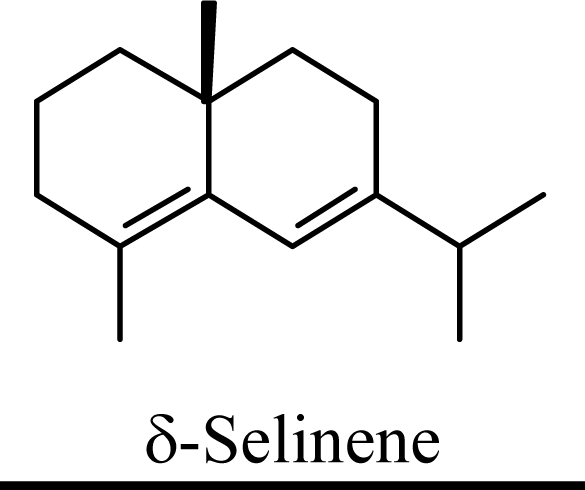 | 0 | 0 | 0 | 0 | 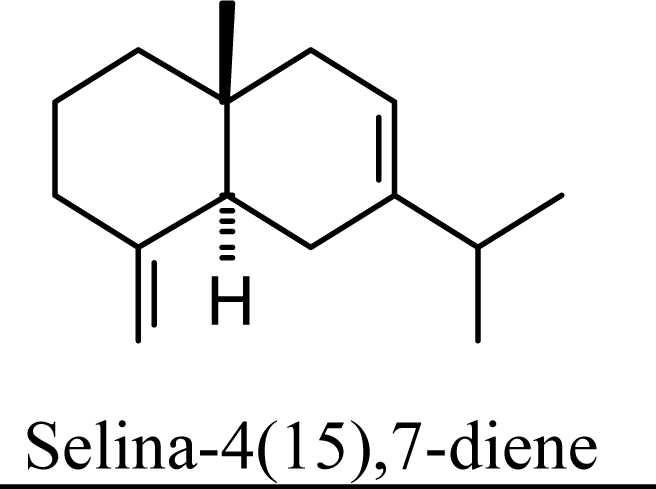 | 14.61 | 14.90 | 13.39 | 13.71 |
| Carbocation | Relative Energies (kcal/mol) | Carbocation | Relative Energies (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B3LYP/6-31G* | MP2/6-31G** | B3LYP/6-31G* | MP2/6-31G** | ||||||
| H(0K) | G° | H(0K) | G | H(0K) | G° | H(0K) | G | ||
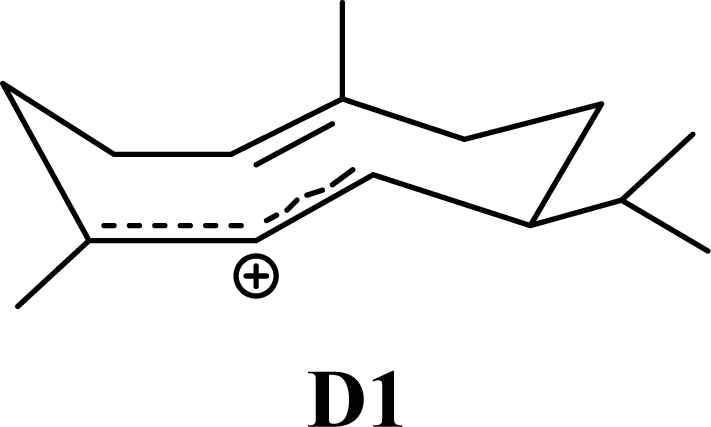 | 4.35 | 4.29 | 7.40 | 6.81 | 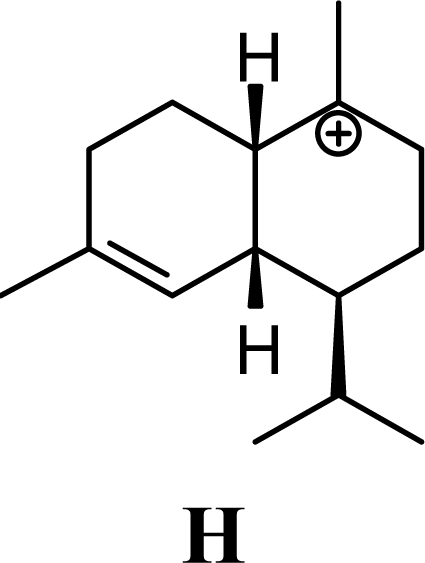 | 1.68 | 1.40 | 1.55 | 1.51 |
 | 5.00 | 4.52 | 7.21 | 6.81 | 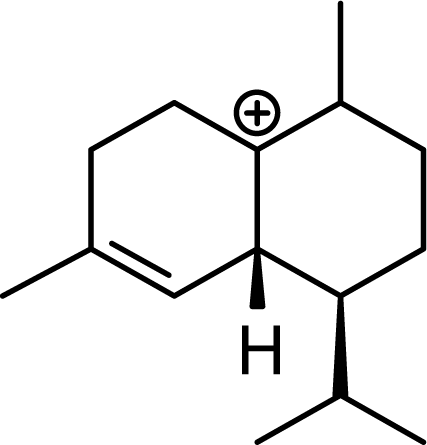 | 0 | 0 | 0 | 0 |
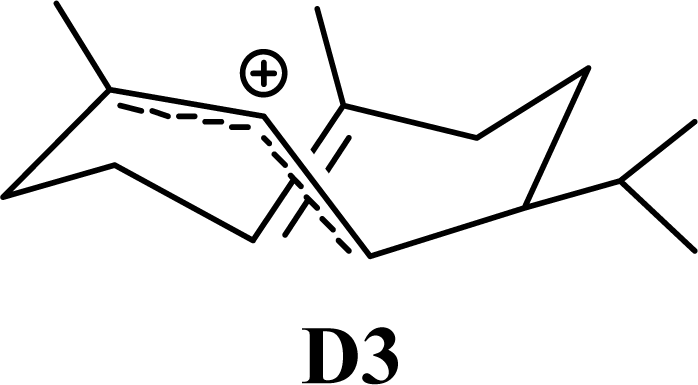 | 5.60 | 5.77 | 7.75 | 7.19 |  | 8.15 | 8.42 | 8.19 | 8.42 |
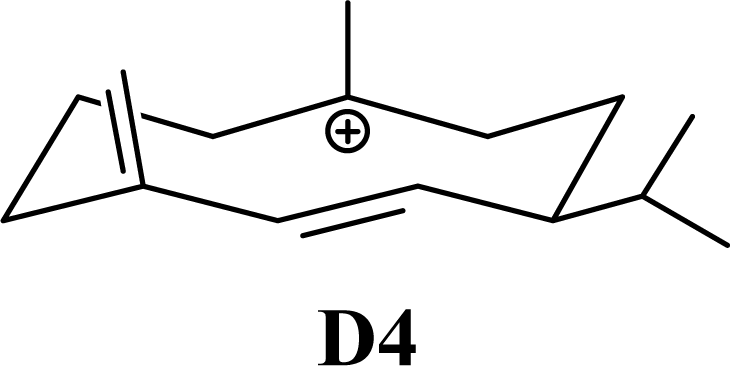 | 15.19 | 15.45 | 11.94 | 11.58 | 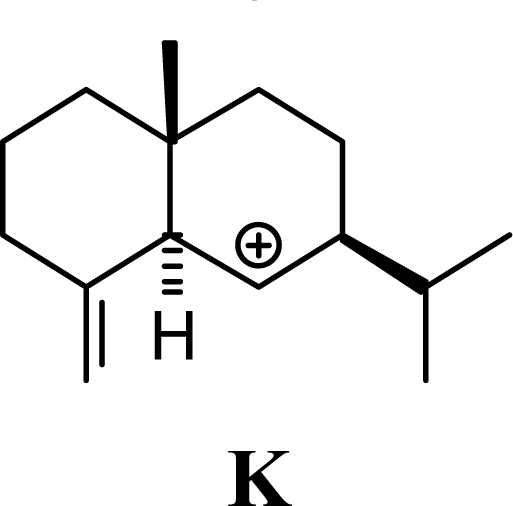 | 26.33 | 26.08 | 22.31 | 22.95 |
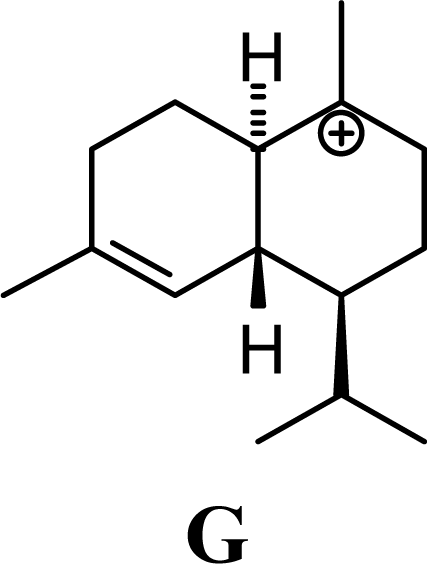 | 2.43 | 1.92 | 1.94 | 1.92 | 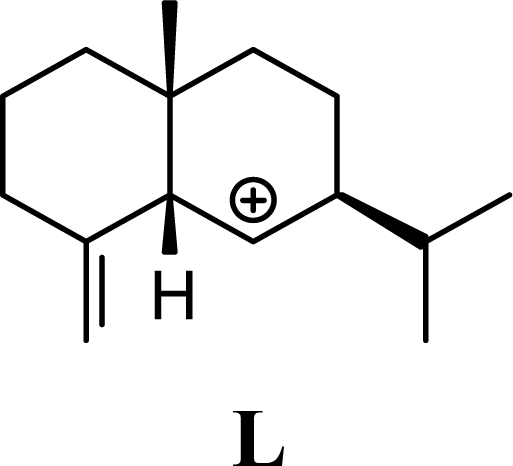 | 23.05 | 23.23 | 18.91 | 19.06 |
Share and Cite
Setzer, W.N. Germacrene D Cyclization: An Ab Initio Investigation. Int. J. Mol. Sci. 2008, 9, 89-97. https://doi.org/10.3390/ijms9010089
Setzer WN. Germacrene D Cyclization: An Ab Initio Investigation. International Journal of Molecular Sciences. 2008; 9(1):89-97. https://doi.org/10.3390/ijms9010089
Chicago/Turabian StyleSetzer, William N. 2008. "Germacrene D Cyclization: An Ab Initio Investigation" International Journal of Molecular Sciences 9, no. 1: 89-97. https://doi.org/10.3390/ijms9010089
APA StyleSetzer, W. N. (2008). Germacrene D Cyclization: An Ab Initio Investigation. International Journal of Molecular Sciences, 9(1), 89-97. https://doi.org/10.3390/ijms9010089





