Abstract
In this work we present results about the deuterium isotope effect on the global kinetics of a Belousov-Zhabotinsky reaction in batch conditions. A nonlinear dependence of the Induction Period upon the percentage of deuterated reactants was found. The isotopic effect on the bromination reaction of malonic acid was evaluated.
1. Introduction
The Belousov-Zhabotinsky (BZ) reaction represents the most famous example of chemical oscillator [1]. Briefly it consists of a catalytic oxidation of an organic substrate with active methylenic groups, by bromate ions in acidic aqueous solution. The most used substrate is malonic acid (MA), catalysts are generally metal redox couples (Ce(IV)/Ce(III), Mn(III)/Mn(II)) or metallo-complexes (ferroin, Ru(bpy)32+).
From 1972 various detailed kinetic models have been proposed to explain the oscillatory behavior of the BZ reaction. The first and simplest is known as the FKN [2], which involves 18 elementary steps and 21 chemical species but can be simplified considering the role of three key species: HBrO2 as exchange intermediate, Br− as control intermediate and Mox, i.e. the oxidized form of the catalyst, as regeneration intermediate. Successively other models have been proposed to improve or fix some aspects of the FKN mechanism: the radicalator model [3], the GTF model [4] and the MBM model [5].
Isotopic effect due to the Hydrogen substitution by Deuterium is a subject of widespread interest among the chemical kinetics discipline. Several organic and inorganic reactions (enolization, substitution, exchange) have been studied in the past for a large class of compounds. The implications of the H-D substitution are also important for the kinetics and the rate of more complex reaction mechanisms. As an example, in the past, the kinetics of the isotope exchange reactions of RCH(COOH)2 (R=H, D, Me, Et, Bu, and Ph) in D2O solutions were studied by using 1H NMR spectroscopy, and the implications for the BZ reaction have been discussed [6].
The influence of deuterated compounds on the global kinetics of BZ reaction was discussed by Karavaev et al. for Ru-catalyzed systems [7]. It was found that the substitution of H2O by D2O as solvent caused a complete suppression of the oscillating regime.
In our study we found a nonlinear increment of the Induction Period (IP) upon the addiction of deuterium substituted reactants for a Ce-catalyzed BZ system.
The Induction Period is the preoscillatory stage in which [Br−] is increased and brominated organic species are accumulated. According to the GTF model [4] the length of IP is determined by the concentration of bromomalonic acid (BrMA). The crucial amount of BrMA necessary for the onset of oscillations is produced through two main pathways: (a) the bromination of the enol form of malonic acid by Br2 and (b) the reaction between Br2 and the malonyl radical formed in the Ce-MA subsystem [8]. Previous works showed a direct relationship between the enolization constant of different organic substrates in BZ-like oscillating systems and the length of the IP [9–11].
In this paper we evaluate the effect of hydrogen-deuterium substitution for reaction pathway (a).
2. Experimental Section
Isotopic effect on bromination reaction was evaluated following changes in the bromine concentration with a double beam spectrophotometer (ULTROSPEC 2000 UV-VIS, Pharmacia Biotech) at λ = 500 nm, where Br2 has an absorption shoulder and its molar extinction coefficient ɛ is 37.5 M−1 cm−1. Measures were performed in thermostated quartz cuvettes (1 × 1 × 4.5 cm) at 20 °C. The spectrophotometer was connected to a PC for data storage and treatment. All reagents were of analytical grade (SIGMA) and they were used without further purifications. The following stock solutions were prepared: BrO3−/H2(D2)SO4 0.5 M, Br− 0.97 M and malonic acid (MA) 0.5 M, reagents were dissolved in double distilled water and/or 99.98% D2O (SIGMA). Effective deuteration of the methylenic group of MA was checked by 1H NMR measurements, which showed a signal at δ = 3.388 ppm corresponding to the CH2 and a signal at δ = 3.247 ppm corresponding to CHD for deuterated solutions. The intensity of the signal was proportional to the percentage of D2O used to dissolve MA.
Solutions at known bromine concentrations were prepared according to the following reaction:
All solutions were prepared in stoichiometric proportions immediately before each spectrophotometric run. Solutions at different known [Br2] were used to calibrate the instrument both in H2O and D2O.
Measurements of BZ Induction Period were performed with a double beam spectrophotometer (Varian, 634 series), in thermostated quartz cuvettes (1 × 1 × 4 cm) at 20 °C following the behavior of Ce(IV) at λ = 320 nm. BZ oscillators in H2O and D2O were prepared using following stock solutions: CeSO4. 2H2O 8 × 10−3 M, MA 0.6 M, NaBrO3 0.18 M; each stock solution was 1 M H2SO4 or D2SO4. Reagents were prepared both in double distilled water and 99.98% D2O (SIGMA). BZ systems were prepared mixing normal and deuterated reagents in different proportions, in order to get a final composition of [MA]t = 0.1 M, [BrO3−]t = 3 × 10−2 M, [Ce]t = 1.3 × 10−3 M and 1 M H2 (D2)SO4, where [X]t indicates the sum of deuterated and normal reagents.
3. Results and Discussion
Figure 1 shows the comparison between a BZ reaction with fully deuterated reagents (Figure 1a) and a BZ reaction in H2O (Figure 1b). The effect of deuterium substitution is not as dramatic as in the Ruthenium catalyzed BZ systems, where oscillations are completely damped [7], nevertheless the global kinetics of the reaction results slower. Addition of deuterated reactants does not alter the qualitative profile of the absorbance as a function of time but influences the oscillatory parameters. This fact is particularly evident from a markedly longer Induction Period, from 150 seconds in H2O to 390 seconds in D2O.
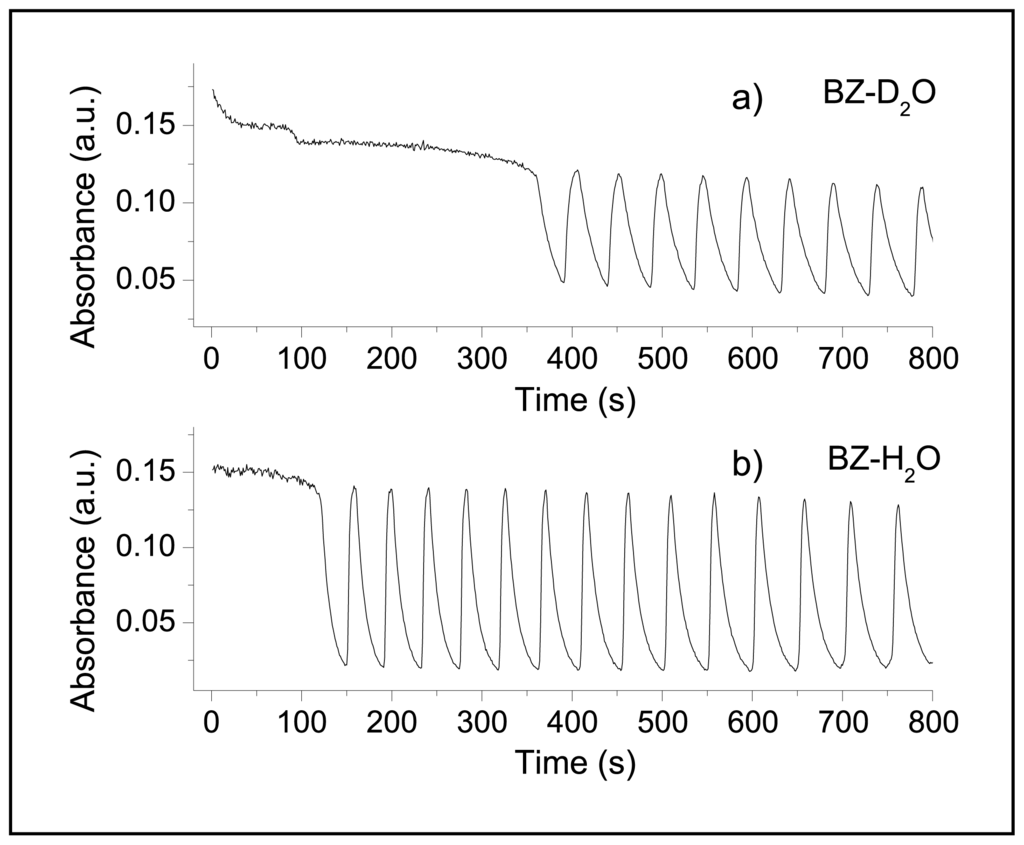
Figure 1.
Comparison between a BZ reaction in D2O (a) and in H2O (b) at 20 °C. Temporal series were recorded at λ = 320 nm where Ce(IV) has an absorption maximum. For both systems the following reactants concentrations were used: [MA] = 0.1 M, [BrO3−] = 3 × 10−2 M, [H2SO4] = 1 M and [Ce] = 1.3 × 10−3 M.
Kreuels et al. [12] found a linear D2O dosage dependence of the oscillation frequency (OF) both at low (increased OF) and high (decreased OF) catalyst concentration. In our experimental condition the oscillation frequency was found substantially independent from D2O dosage, in fact it changes from 0.023 Hz in H2O to 0.021 Hz for fully deuterated reagents.
On the contrary, respect to data on the oscillation frequency, a nonlinear response of IP to D2O content was found. The dependence of IP upon the percentage of deuterated reactants present in solution is shown in Figure 2. The monotonic increase of IP is in line with an expected isotopic effect, which generally slows down rates of elementary reactions with proton involvement [13].
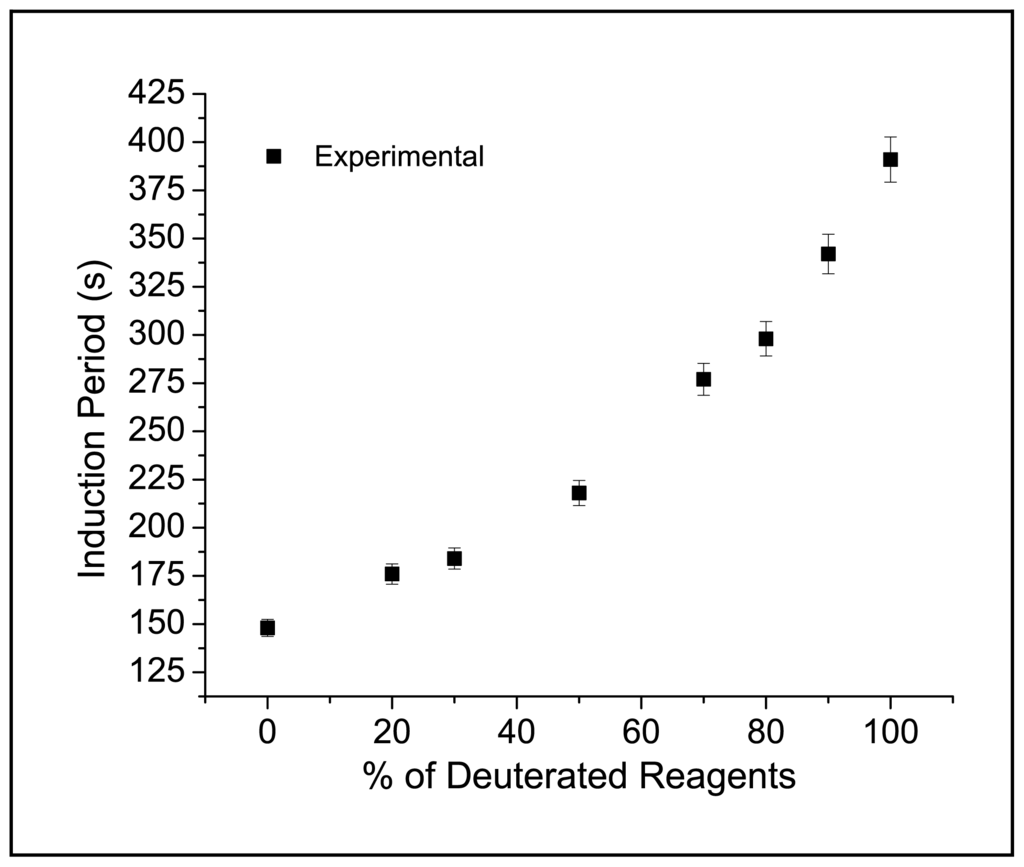
Figure 2.
Dependence of Induction Period upon percentage of deuterated reactants at 20 °C for BZ reactions with the following composition [MA] = 0.1 M, [BrO3−] = 3 × 10−2 M, [H2SO4] = 1 M and [Ce] = 1.3 × 10−3 M.
As stated before, to understand the IP behavior we investigated the bromination reaction of malonic acid through its enolic form. For practical purposes this study reduces to the investigation of the ketoenolic equilibrium, which is the rate determinant step [14,15]. According to the GTF model, the bromination proceeds as follows:
Where CH2(COOH)2 is the keto form (MA) and (COOH)CHC(OH)2 is the enolic form (ENOL).
It has been shown [15] that HOBr in reaction (4) does not compete with Br2, hence the kinetics of bromination is well described by steps (2), (3) and (5). In particular we were interested in the first two steps. The global reaction rate can be written in terms of Br2 disappearance [14]:
From equations (2) and (3) we know that:
We can consider some approximations: in strong acidic medium (0.05 M < [H2SO4] <1 M) [MA] is approximatively equal to the analytic concentration [MA]0 and being k2 >> k1[16], the variation of enol concentration with time is close to 0 (steady state approximation) [14]:
It follows that:
Substituting in (6):
Finally, in our experimental condition we know that k2[Br2] >> k−1, so at the end we get the operative equation to calculate enolization rate constant:
Equation (11) describes a pseudo zero order kinetics.
Figure 3 shows the absorbance plots of Br2 against time during the halogenation of malonic acid in D2O (Figure 3a) and H2O (Figure 3b). The linear trend of Br2 disappearance confirmed an effective zero order reaction both in H2O and D2O solutions. In our experiments we used [MA] = 0.25 M for all spectrophotometric runs and we varied [Br2] between 7.5 × 10−3 M and 5.2 × 10−2 M, Br2 was produced in situ according to reaction (1).
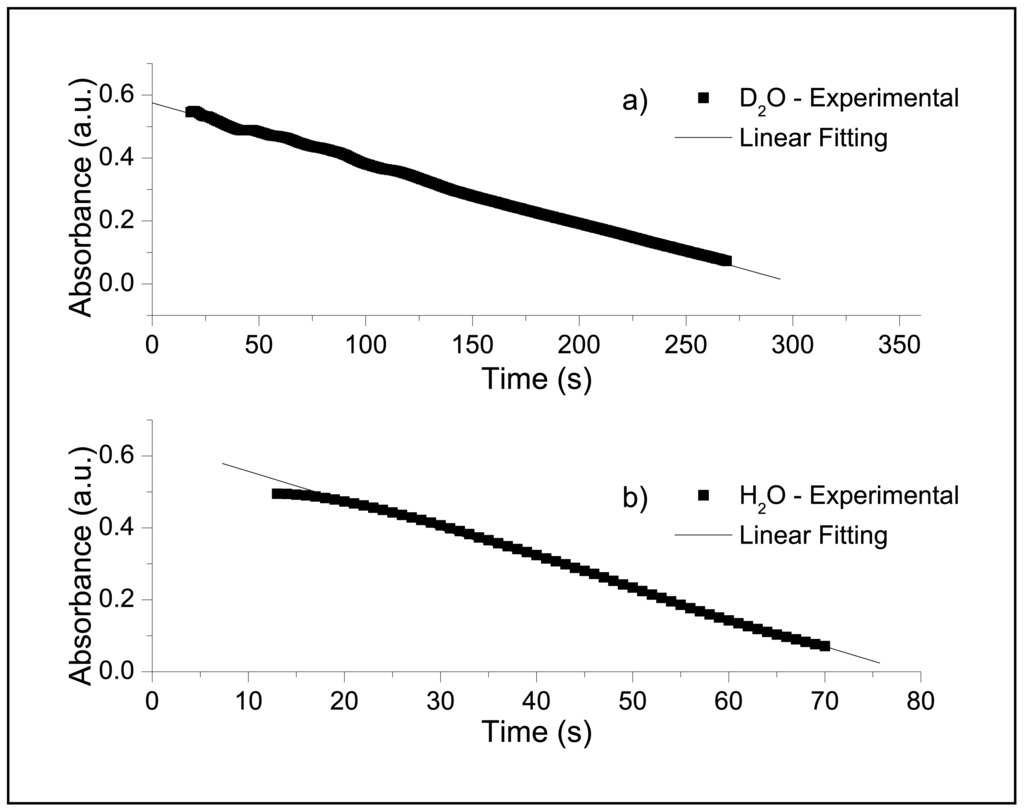
Figure 3.
Disappearance of Br2 followed spectrophotometrically at 500 nm in H2O syatem (a) and D2O system (b). Solution composition was [MA] = 0.25 M, [Br2] = 7.5 × 10−3 M.
Using calibration curves and equation (11) we finally could calculate enolization constants from spectrophotometric data for H2O system (k1h = 2.025 × 10−3 s−1, slightly lower than that reported in literature at 25 °C, 3 × 10−3 s−1) and D2O system (k1d = 2.666 × 10−4 s−1). The value of the ratio k1h/k1d = 7.6 suggests a primary isotopic effect [13] due to the D-H substitution of the methylenic hydrogens of malonic acid.
In conclusion, we showed how the progressive substitution of deuterated reactants in a Cerium catalyzed BZ reaction caused an increase in the Induction Period length. Data on the bromination reaction revealed a primary isotopic effect on the enolization kinetic constant of malonic acid, which in turn determined a slower production of bromomalonic acid contributing to a delayed onset of oscillations. In order to fully understand modifications induced by deuterated reagents to the BZ system, also the BrMA production occurring through the reaction between Br2 and the malonyl radical should be investigated. A deeper investigation can result in a better understanding of experimental data reported in Figure 2.
Acknowledgements
Thanks are due to R. Lombardo and S. Ristori for fruitful discussions and to A. Donati and E. Simoncini for experimental help.
References and Notes
- Taylor, A.F. Mechanism and Phenomenology of an Oscillating Chemical Reaction. Prog. Reac. Kin. Mech 2002, 27, 247–325. [Google Scholar]
- Field, R.J.; Korös, E.; Noyes, R.M. Oscillations in chemical systems. II. Thorough analysis of temporal oscillation in the bromate-cerium-malonic acid system. J. Am. Chem. Soc 1972, 94, 8649–8664. [Google Scholar]
- Försterling, H.D.; Muranyi, S.; Noszticzius, Z. Evidence of malonyl radical controlled oscillations in the Belousov-Zhabotinskii reaction (malonic acid-bromate-cerium system). J. Phys. Chem 1990, 94, 2915–2921. [Google Scholar]
- Gyorgyi, L.; Turanyi, T.; Field, R.J. Mechanistic details of the oscillatory Belousov-Zhabotinskii reaction. J. Phys. Chem 1990, 94, 7162–7170. [Google Scholar]
- Hegedus, L.; Wittman, M.; Noszticzius, Z.; Yan, S.H.; Sirimungkala, A.; Försterling, H.D.; Field, R.J. HPLC analysis of complete BZ systems. Evolution of the chemical composition in cerium and ferroin catalysed batch oscillators: experiments and model calculations. Faraday Discuss 2001, 120, 21–38. [Google Scholar]
- Hsu, M.C.; Jwo, J.J. Kinetic study of the isotope exchange reactions of malonic acid and its derivatives in various acidic D2O media using 1H NMR spectroscopy. Implication in the Belousov-Zhabotinsky reaction. Int. J. Chem. Kin 1999, 31, 455–461. [Google Scholar]
- Karavaev, A.D.; Kazakov, V.P.; Tolstikov, G.A. Deuteration Effect in Auto-Oscillation Chemiluminescence of the Belousov-Zhabotinskii Reaction. React. Kinet. Catal. Lett 1986, 32, 21–26. [Google Scholar]
- Cadena, A.; Pérez, N.; Ágreda, J.A.; Barragán, D. Understanding the Induction Period of the Belousov-Zhabotinsky Reaction. J. Braz. Chem. Soc 2005, 16, 214–219. [Google Scholar]
- Berenstein, I.; Agreda, J.; Barragán, D. Induction Period in the BrO3−, Ce(III), H2SO4, oxalic acid and ketone oscillating reaction. Phys. Chem. Chem. Phys 1999, 1, 4601–4603. [Google Scholar]
- Berenstein, I.; Agreda, J.; Barragán, D. Effect of Methyl Ketones in the Belousov-Zhabotinskii Reaction. J. Phys. Chem. A 1999, 103, 9780–9782. [Google Scholar]
- Berenstein, I.; Barragán, D. Additive Effects of Methyl Ketones in the Belousov-Zhabotinsky Reactions. J. Braz. Chem. Soc 2004, 15, 844–848. [Google Scholar]
- Kreuels, T.; Martin, W.; Brinkmann, K. Influence of D2O on the Belousov-Zhabotinsky Reaction. Ber. Bunsenges. Phys. Chem 1980, 84, 411–412. [Google Scholar]
- Collins, C.J.; Bowman, N.S. (Eds.) Isotope Effects in Chemical Reactions; ACS Monograph No. 167; Van Nostrand Reinhold: New York, 1970.
- Sirimungkala, A.; Försterling, H.D.; Dlask, V.; Field, R.J. Bromination Reactions Important in the Mechanism of the Belousov-Zhabotinsky System. J. Phys. Chem. A 1999, 103, 1038–1043. [Google Scholar]
- Sevcík, P.; Snirková, M.; Hlavácová, J. Kinetics and Simulations of the Malonic Acid-Bromine Reaction. React. Kinet. Catal. Lett 1994, 52, 161–168. [Google Scholar]
- Edelson, D; Noyes, R.M.; Field, R.J. Mechanistic Details of the Belousov-Zhabotinsky Oscillations. II. The Organic Reaction Subset. Int. J. Chem. Kin 1979, 11, 155–164. [Google Scholar]
© 2007 by MDPI Reproduction is permitted for noncommercial purposes.