Salting-out in Aqueous Solutions of Ionic Liquids and K3PO4: Aqueous Biphasic Systems and Salt Precipitation
Abstract
:1. Introduction
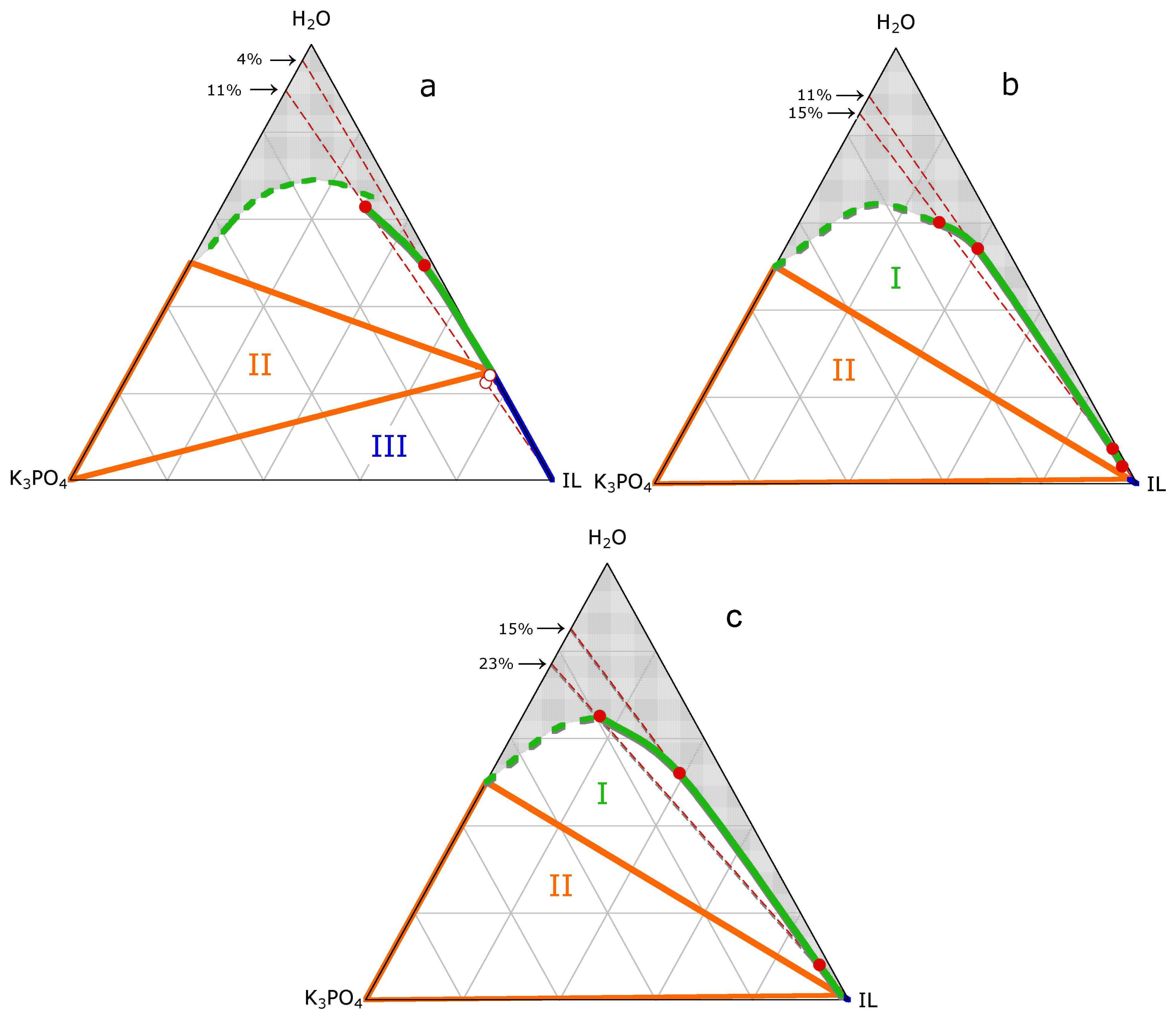
2. Results
3. Discussion
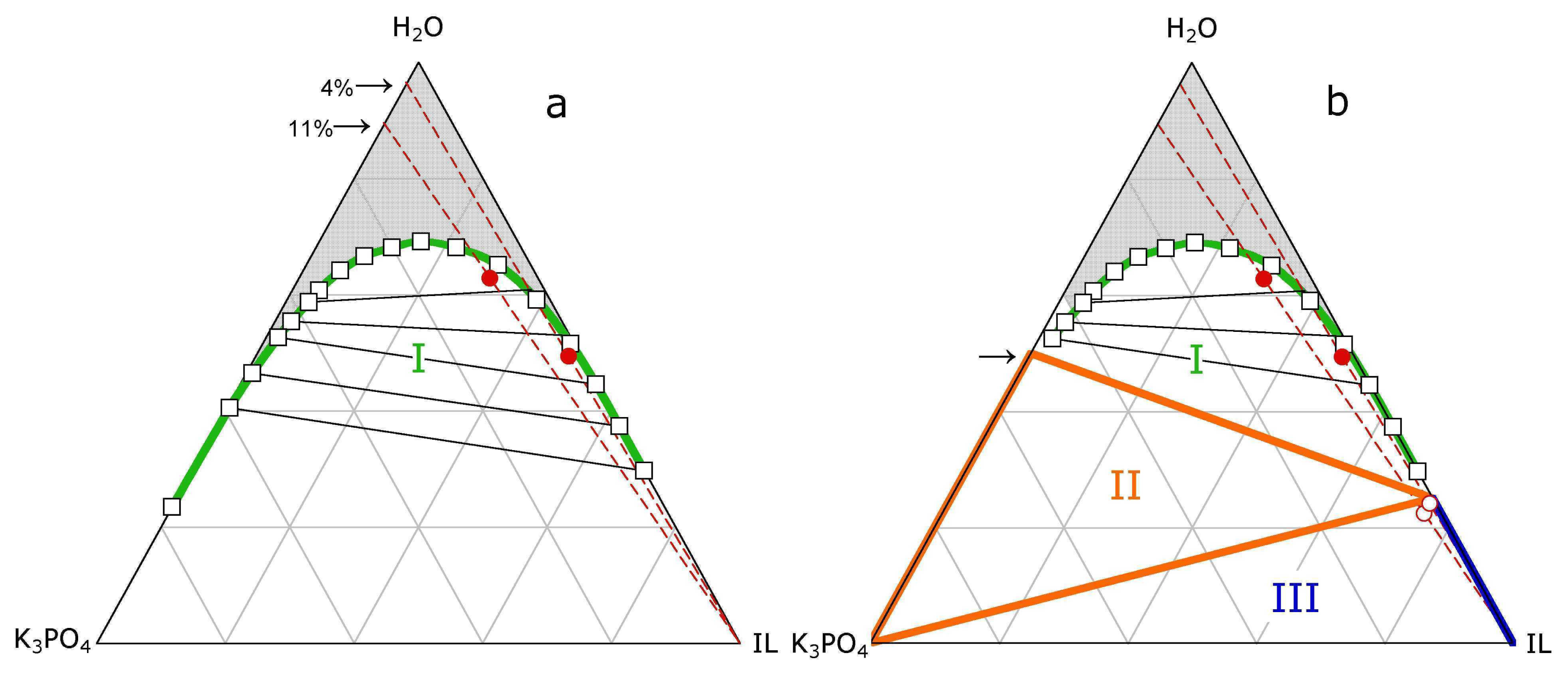
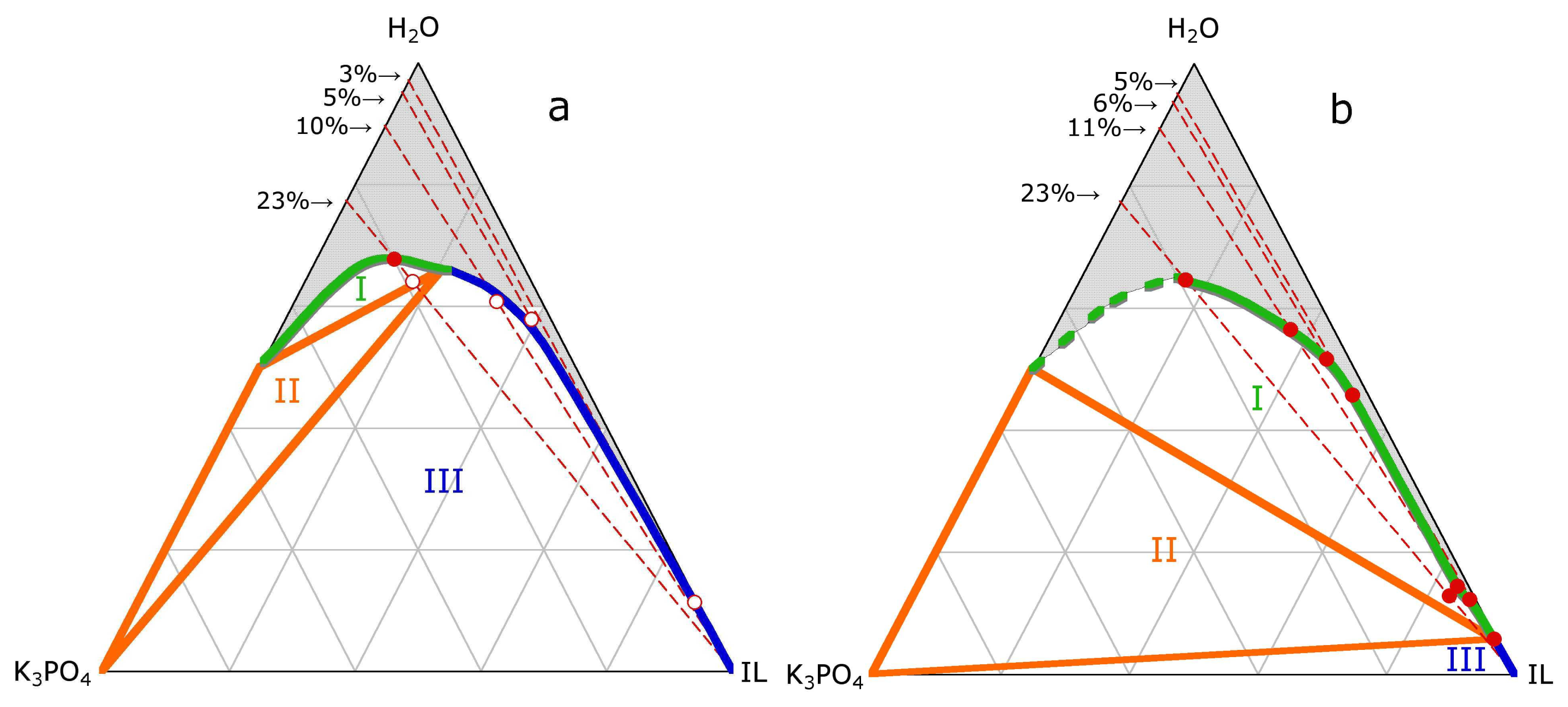
3.1. ABS and precipitation
3.2. ABS and Micelle formation
4. Conclusions
5. Experimental Section
5.1. Chemicals and Preparation of Solutions
5.2. Experimental procedure
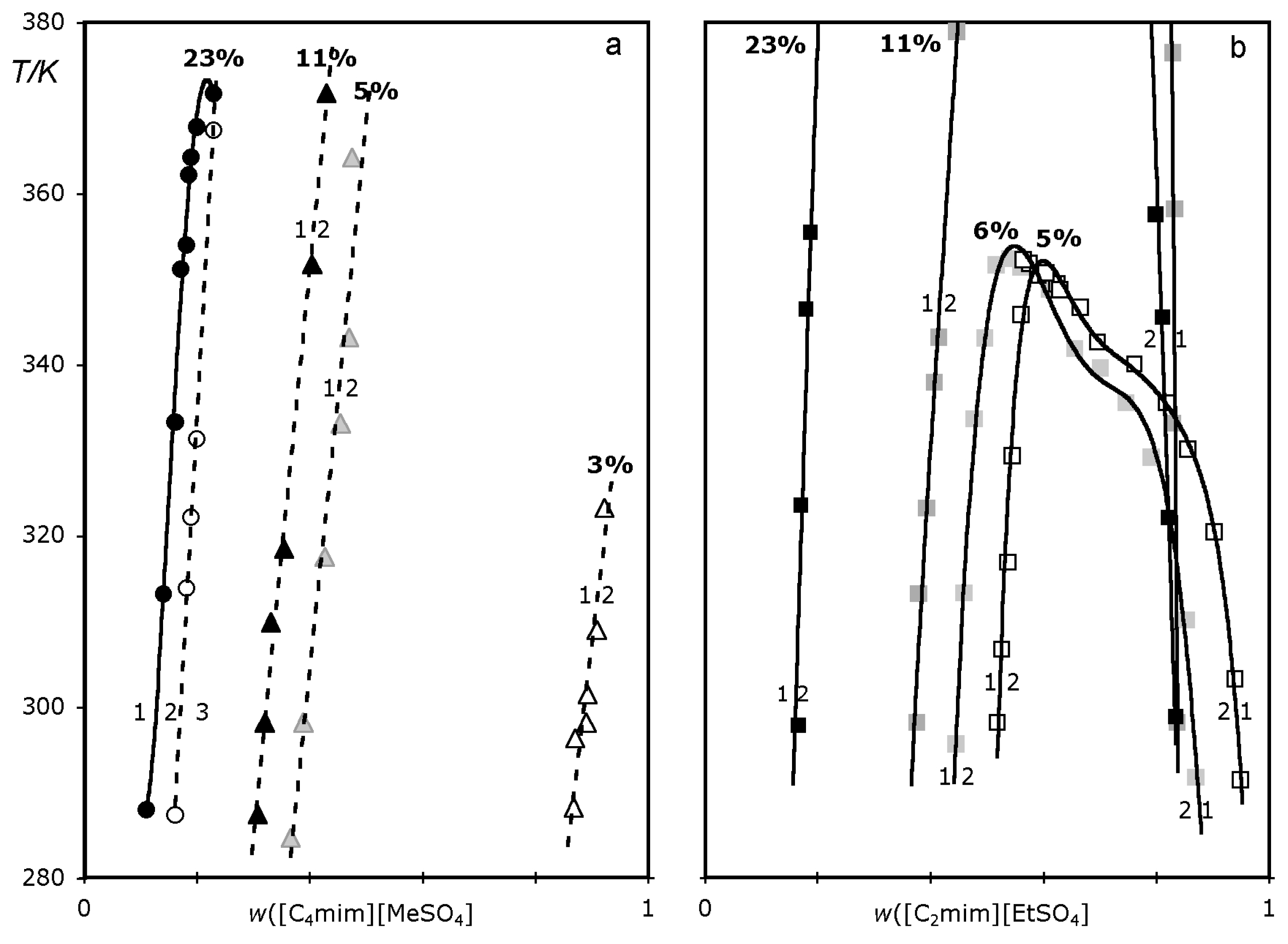
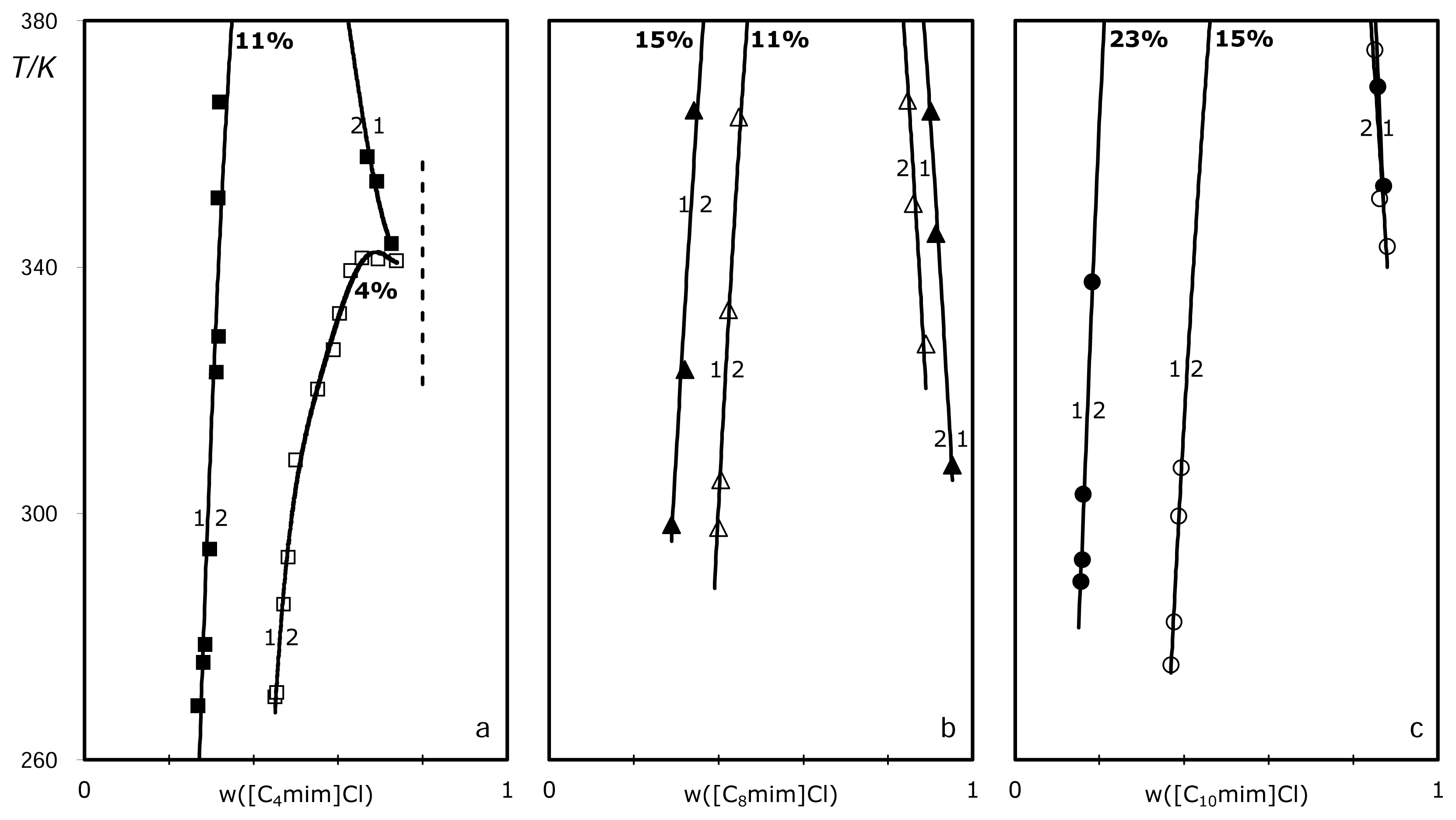
| wsalt,B | wsa | wIL, T | T/K | wsalt,T | wIL, T | T/K | |
|---|---|---|---|---|---|---|---|
| K3PO4 (salt) + [C2mim][EtSO4] (IL) + H2O | |||||||
| 0.04889 | 0.02355 | 0.51828 | 298.2 | 0.01815 | 0.62886 | 348.7 | |
| 0.02321 | 0.52537 | 306.7 | 0.01636 | 0.66545 | 346.7 | ||
| 0.02266 | 0.53650 | 316.9 | 0.01489 | 0.69548 | 342.6 | ||
| 0.02226 | 0.54476 | 329.3 | 0.01165 | 0.76169 | 340.0 | ||
| 0.02150 | 0.56029 | 345.8 | 0.00881 | 0.81977 | 335.5 | ||
| 0.02134 | 0.56350 | 352.2 | 0.00703 | 0.85617 | 330.1 | ||
| 0.02078 | 0.57505 | 351.8 | 0.00474 | 0.90309 | 320.4 | ||
| 0.02005 | 0.59000 | 350.4 | 0.00295 | 0.93973 | 303.2 | ||
| 0.01934 | 0.60442 | 350.7 | 0.00248 | 0.94931 | 291.4 | ||
| 0.01840 | 0.62356 | 349.4 | |||||
| 0.06313 | 0.03500 | 0.44551 | 295.6 | 0.02465 | 0.60950 | 348.8 | |
| 0.03410 | 0.45986 | 313.2 | 0.02179 | 0.65482 | 341.8 | ||
| 0.03294 | 0.47820 | 333.6 | 0.01891 | 0.70039 | 339.5 | ||
| 0.03180 | 0.49622 | 343.1 | 0.01599 | 0.74670 | 335.6 | ||
| 0.03057 | 0.51581 | 351.6 | 0.01311 | 0.79229 | 329.2 | ||
| 0.02903 | 0.54018 | 352.4 | 0.00915 | 0.85511 | 310.1 | ||
| 0.02774 | 0.56056 | 351.3 | 0.00820 | 0.87018 | 291.7 | ||
| 0.10538 | 0.06571 | 0.37641 | 298.2 | 0.05824 | 0.44737 | 378.9 | |
| 0.06537 | 0.37970 | 313.2 | 0.01791 | 0.83000 | 333.2 | ||
| 0.06386 | 0.39397 | 323.2 | 0.01790 | 0.83018 | 376.4 | ||
| 0.06241 | 0.40774 | 337.9 | 0.01756 | 0.83333 | 358.2 | ||
| 0.06165 | 0.41500 | 343.2 | 0.01715 | 0.83721 | 298.2 | ||
| 0.22670 | 0.18891 | 0.16610 | 297.8 | 0.03732 | 0.83526 | 298.8 | |
| 0.18791 | 0.17051 | 323.5 | 0.04023 | 0.82240 | 322.1 | ||
| 0.18595 | 0.17919 | 346.4 | 0.04259 | 0.81201 | 345.5 | ||
| 0.18402 | 0.18769 | 355.4 | 0.04534 | 0.79986 | 357.5 | ||
| K3PO4 (salt) + [C4mim][MeSO4] (IL) + H2O | |||||||
| 0.02919 | 0.00389 | 0.86667 | 288.2 | 0.00317 | 0.89130 | 301.5 | |
| 0.00379 | 0.87013 | 296.3 | 0.00269 | 0.90794 | 309.0 | ||
| 0.00321 | 0.88989 | 298.2 | 0.00231 | 0.92099 | 323.2 | ||
| 0.04880 | 0.03094 | 0.36604 | 284.7 | 0.02668 | 0.45326 | 333.2 | |
| 0.02984 | 0.38859 | 298.2 | 0.02594 | 0.46850 | 343.2 | ||
| 0.02803 | 0.42558 | 317.5 | 0.02565 | 0.47445 | 364.2 | ||
| 0.10538 | 0.07298 | 0.30741 | 287.4 | 0.06799 | 0.35484 | 318.4 | |
| 0.07161 | 0.32044 | 298.2 | 0.06287 | 0.40339 | 351.7 | ||
| 0.07057 | 0.33036 | 309.9 | 0.06022 | 0.42857 | 371.8 | ||
| 0.22670 | 0.20189 | 0.10944 | 287.9 | 0.18455 | 0.18592 | 362.2 | |
| 0.19496 | 0.14000 | 313.2 | 0.18368 | 0.18975 | 364.2 | ||
| 0.19012 | 0.16137 | 333.3 | 0.18368 | 0.18975 | 322.2 | ||
| 0.19012 | 0.16137 | 287.4 | 0.18148 | 0.19947 | 367.8 | ||
| 0.18786 | 0.17132 | 351.2 | 0.18148 | 0.19947 | 331.4 | ||
| 0.18555 | 0.18152 | 354.0 | 0.17465 | 0.22962 | 371.7 | ||
| 0.18555 | 0.18152 | 313.8 | 0.17465 | 0.22962 | 367.4 | ||
| wsalt,B | wsalt,T | wIL, T | T/K | wsalt,T | wIL, T | T/K |
|---|---|---|---|---|---|---|
| K3PO4 (salt) + [C4mim]Cl (IL) + H2O | ||||||
| 0.03604 | 0.01979 | 0.45086 | 270.2 | 0.01480 | 0.58942 | 326.6 |
| 0.01964 | 0.45522 | 270.9 | 0.01429 | 0.60341 | 332.5 | |
| 0.01904 | 0.47167 | 285.2 | 0.01332 | 0.63045 | 339.4 | |
| 0.01865 | 0.48246 | 292.8 | 0.01237 | 0.65672 | 341.5 | |
| 0.01802 | 0.49992 | 308.7 | 0.01098 | 0.69536 | 341.3 | |
| 0.01617 | 0.55142 | 320.2 | 0.00942 | 0.73873 | 341.0 | |
| 0.10538 | 0.07703 | 0.26904 | 268.7 | 0.07193 | 0.31744 | 328.8 |
| 0.07572 | 0.28143 | 275.8 | 0.07167 | 0.31986 | 366.8 | |
| 0.07522 | 0.28625 | 278.7 | 0.03487 | 0.66915 | 357.9 | |
| 0.07413 | 0.29654 | 294.1 | 0.03250 | 0.69161 | 353.9 | |
| 0.07248 | 0.31223 | 322.9 | 0.02884 | 0.72634 | 343.8 | |
| 0.07197 | 0.31707 | 351.2 | ||||
| K3PO4 (salt) + [C8mim]Cl (IL) + H2O | ||||||
| 0.10538 | 0.06320 | 0.40022 | 297.7 | 0.01626 | 0.84566 | 367.1 |
| 0.06265 | 0.40548 | 305.5 | 0.01478 | 0.85979 | 350.4 | |
| 0.06079 | 0.42314 | 333.2 | 0.01164 | 0.88952 | 327.6 | |
| 0.05823 | 0.44747 | 364.4 | ||||
| 0.15324 | 0.10905 | 0.28839 | 298.2 | 0.01519 | 0.90090 | 365.4 |
| 0.10405 | 0.32098 | 323.4 | 0.01329 | 0.91324 | 345.5 | |
| 0.10086 | 0.34181 | 365.5 | 0.00730 | 0.95238 | 307.9 | |
| K3PO4 (salt) + [C10mim]Cl (IL) + H2O | ||||||
| 0.15324 | 0.09676 | 0.36859 | 275.4 | 0.02280 | 0.85121 | 375.3 |
| 0.09547 | 0.37696 | 282.4 | 0.02096 | 0.86320 | 351.1 | |
| 0.09388 | 0.38736 | 299.6 | 0.01824 | 0.88099 | 343.4 | |
| 0.09296 | 0.39339 | 307.4 | ||||
| 0.22670 | 0.19130 | 0.15616 | 289.0 | 0.18526 | 0.18278 | 337.6 |
| 0.19064 | 0.15906 | 292.5 | 0.03221 | 0.85793 | 369.4 | |
| 0.19001 | 0.16186 | 303.1 | 0.02885 | 0.87273 | 353.2 | |
Acknowledgements
References
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddelston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the Aqueous Miscibility of Ionic Liquids: Aqueous Biphasic Systems of Water-Miscible Ionic Liquids and Water-Structuring Salts for Recycle, Metathesis, and Separations. J. Am. Chem. Soc 2003, 125, 6632–6633. [Google Scholar]
- Bridges, N.J.; Gutowski, K.E.; Rogers, R.D. Investigation of Aqueous Biphasic Systems Formed from Solutions of Chaotropic Salts with Kosmotropic Salts (Salt–Salt ABS). Green Chem 2007, 9, 177–183. [Google Scholar]
- Albertsson, P. Partition of Cell Particles and MacromoleculesWiley: New York, 3rd edn; 1986. [Google Scholar]
- Willauer, H.D.; Huddleston, J.G.; Rogers, R.D. Solvent Properties of Aqueous Biphasic Systems Composed of Polyethylene Glycol and Salt Characterized by the Free Energy of Transfer of a Methylene Group between the Phases and by a Linear Solvation Energy Relationship. Ind. Eng. Chem. Res 2002, 41, 2591–2601. [Google Scholar]
- Abraham, M.H.; Zissimos, A.M.; Huddleston, J.G.; Willauer, H.D.; Rogers, R.D.; Acree, W.E., Jr. Some Novel Liquid Partitioning Systems: Water-Ionic Liquids and Aqueous Biphasic Systems. Ind. Eng. Chem. Res 2003, 42, 413–418. [Google Scholar]
- Suarez, P.A.Z.; Einloft, S.; Dullius, J.E.L.; de Souza, R.F.; Dupont, J. Synthesis and Physical Chemical Properties of Ionic Liquids Based on 1-n-butyl-3-methylimidazolium Cation. J. Chim. Phys. Phys.-Chim. Biol 1998, 95, 1626–1639. [Google Scholar]Dullius, J.E.L.; Suarez, P.A.Z.; Einloft, S.; De Souza, R.F.; Dupont, J.; Fisher, J.; Cian, A.D. Selective Catalytic Hydrodimerization of 1,3- butadiene by Palladium Compounds Dissolved in Ionic Liquids. Organometallics 1998, 17, 815–819. [Google Scholar]
- Anthony, J.L.; Maginn, E.J.; Brennecke, J.F. Solution Thermodynamics of Imidazolium-Based Ionic Liquids and Water. J. Phys. Chem. B 2001, 105, 10942–10949. [Google Scholar]
- Trindade, J.R.; Visak, Z.P.; Blesic, M.; Coutinho, J.A.P.; Marrucho, I.M.; Canongia Lopes, J.N.; Rebelo, L.P.N. Salting out effects in aqueous ionic liquid solutions. J. Phys. Chem. B 2007, 111, 4737–4741. [Google Scholar]
- Rebelo, L.P.N.; Najdanovic-Visak, V.; Visak, Z.P.; Nunes da Ponte, M.; Szydlowski, J.; Cerdeiriña, C.A.; Troncoso, J.; Romani, L. A Detailed Thermodynamic Analysis of [C4mim][BF4] + Water as a Case Study to Model Ionic Liquid Aqueous Solutions. Green Chem 2004, 6, 369–381. [Google Scholar]
- Wagner, M.; Stanga, O.; Schröer, W. Corresponding States Analysis of the Critical Points in Binary Solutions of Room Temperature Ionic Liquids. Phys. Chem. Chem. Phys 2003, 5, 3943–3950. [Google Scholar]
- Santos, L.M.N.B.F.; Canongia Lopes, J.N.; Coutinho, J.A.P.; Esperança, J.M.S.S.; Gomes, L.R.; Marrucho, I.M.; Rebelo, L.P.N. Ionic Liquids: First Direct Determination of Their Cohesive Energy. J. Am. Chem. Soc 2007, 129, 284–285. [Google Scholar]
- Rebelo, L.P.N.; Canongia Lopes, J.N.; Esperança, J.M.S.S.; Guedes, H.J.R.; Łachwa, J.; Najdanovic-Visak, V.; Visak, Z.P. Accounting for the Unique, Doubly Dual Nature of Ionic Liquids from a Molecular Thermodynamic and Modeling Standpoint. Acc. Chem. Res. 2007. [Google Scholar] [CrossRef]
- Visak, Z.; Canongia Lopes, J.N.; Rebelo, L.P.N. Ionic Liquids in Polyethylene Glycol Aqueous Solutions: Salting-In and Salting-Out Effects. Monatshefte fur Chemie - Chemical Monthly 2007. [Google Scholar] [CrossRef]
- Grilc, V.; Krašovec, L. Liquid-liquid-solid equilibria for isopropanol-water-alkali phosphate systems. Solvent Extraction and Ion Exchange 1994, 12, 779–788. [Google Scholar]
- Zhao, H.; Song, Z. Nuclear magnetic relaxation of water in ionic-liquid solutions: determining the kosmotropicity of ionic liquids and its relationship with the enzyme enantioselectivity. J. Chem. Technol. Biotechnol 2007, 82, 304–312. [Google Scholar]Tamaki, K.; Ohara, Y.; Isomura, Y. Viscosity B Coefficients for some Alkyl Sulfates in Aqueous Solutions. Bull. Chem. Soc. Japan 1973, 46, 1551–1552. [Google Scholar]
- Blesic, M.; Marquesa, M.H.; Plechkova, N.V.; Seddon, K.R.; Rebelo, L.P.N.; Lopes, A. Self-aggregation of ionic liquids: micelle formation in aqueous solution. Green Chem 2007, 9, 481–490. [Google Scholar]
© 2007 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Najdanovic-Visak, V.; Lopes, J.N.C.; Visak, Z.P.; Trindade, J.; Rebelo, L.P.N. Salting-out in Aqueous Solutions of Ionic Liquids and K3PO4: Aqueous Biphasic Systems and Salt Precipitation. Int. J. Mol. Sci. 2007, 8, 736-748. https://doi.org/10.3390/i8080736
Najdanovic-Visak V, Lopes JNC, Visak ZP, Trindade J, Rebelo LPN. Salting-out in Aqueous Solutions of Ionic Liquids and K3PO4: Aqueous Biphasic Systems and Salt Precipitation. International Journal of Molecular Sciences. 2007; 8(8):736-748. https://doi.org/10.3390/i8080736
Chicago/Turabian StyleNajdanovic-Visak, Vesna, José N. Canongia Lopes, Zoran P. Visak, J. Trindade, and Luís P. N. Rebelo. 2007. "Salting-out in Aqueous Solutions of Ionic Liquids and K3PO4: Aqueous Biphasic Systems and Salt Precipitation" International Journal of Molecular Sciences 8, no. 8: 736-748. https://doi.org/10.3390/i8080736
APA StyleNajdanovic-Visak, V., Lopes, J. N. C., Visak, Z. P., Trindade, J., & Rebelo, L. P. N. (2007). Salting-out in Aqueous Solutions of Ionic Liquids and K3PO4: Aqueous Biphasic Systems and Salt Precipitation. International Journal of Molecular Sciences, 8(8), 736-748. https://doi.org/10.3390/i8080736




