Electrochemical Studies of Camptothecin and Its Interaction with Human Serum Albumin
Abstract
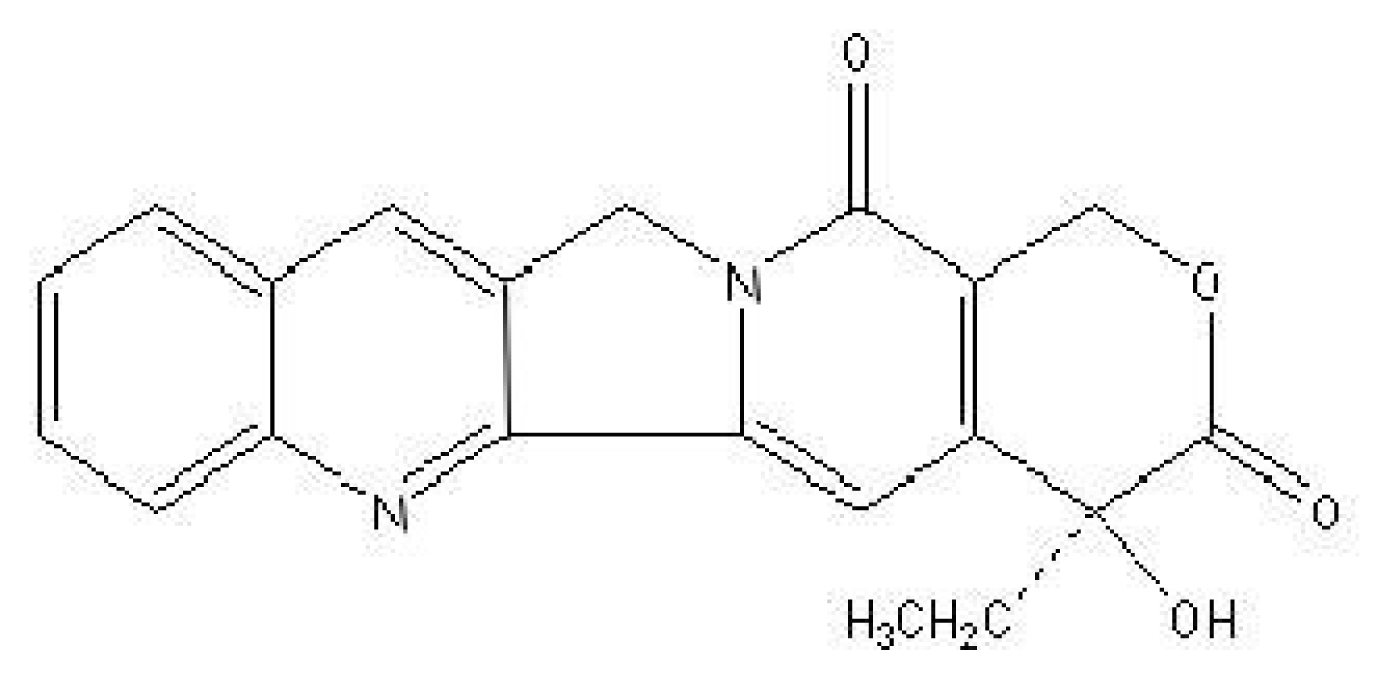
:1. Introduction
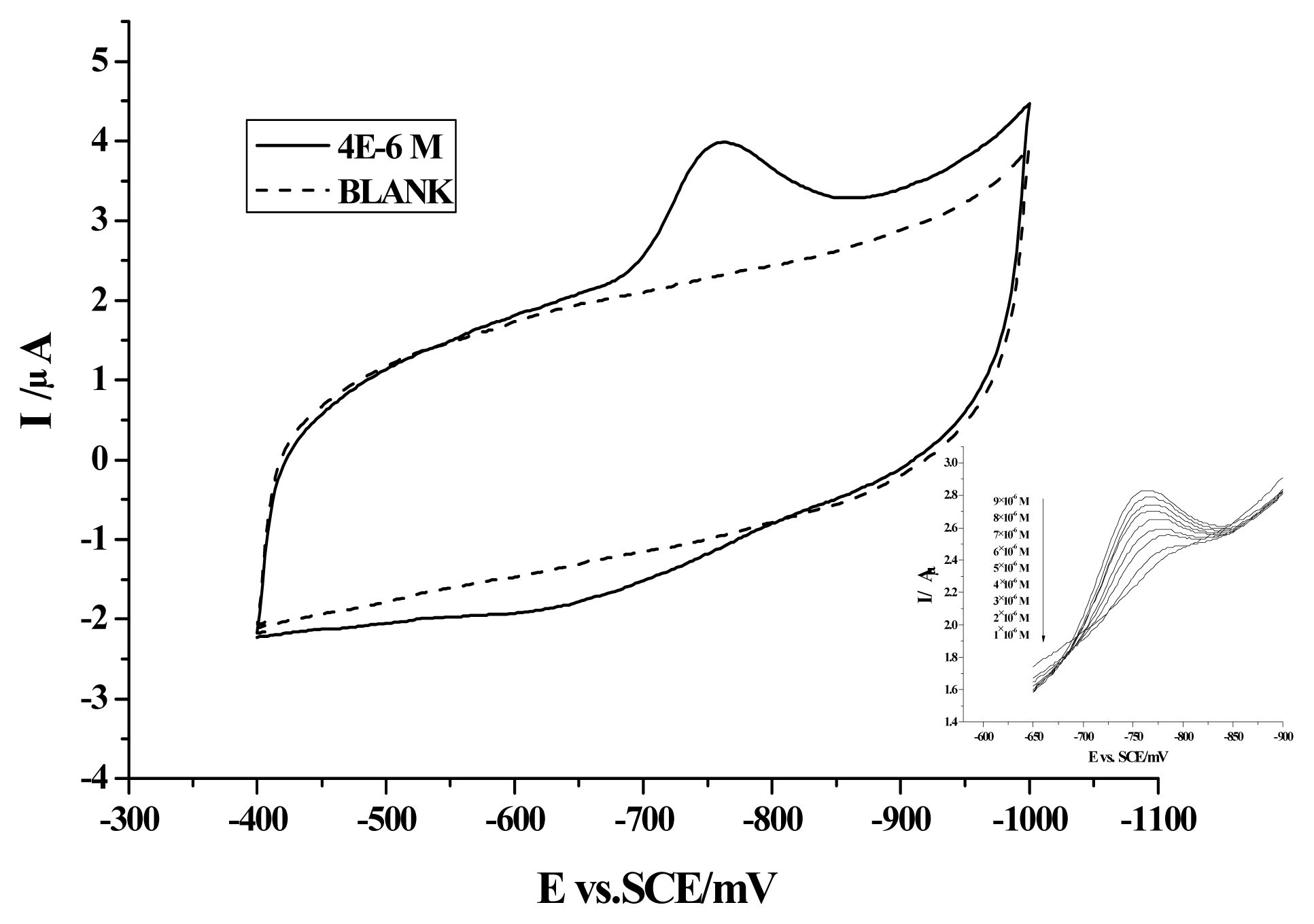
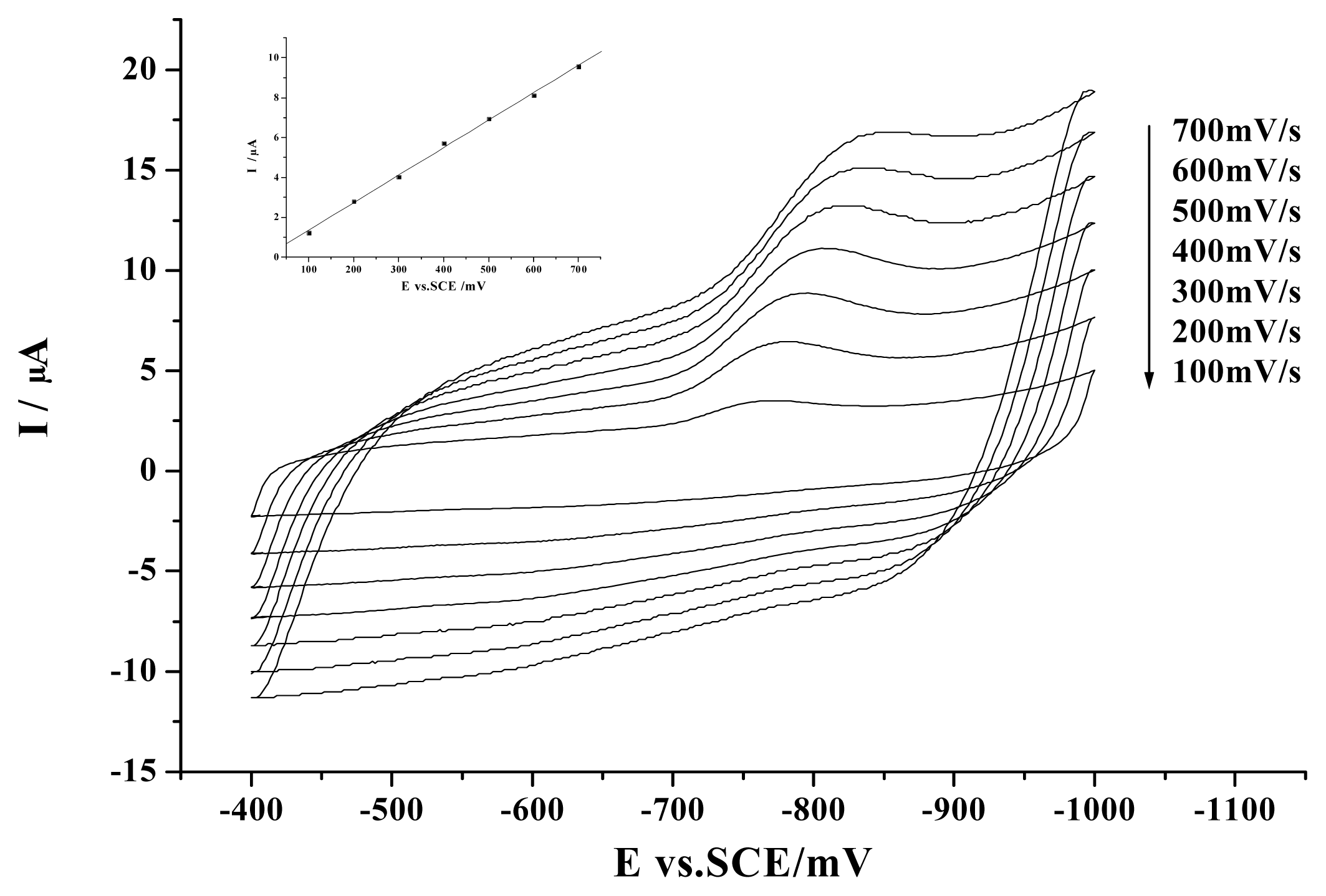
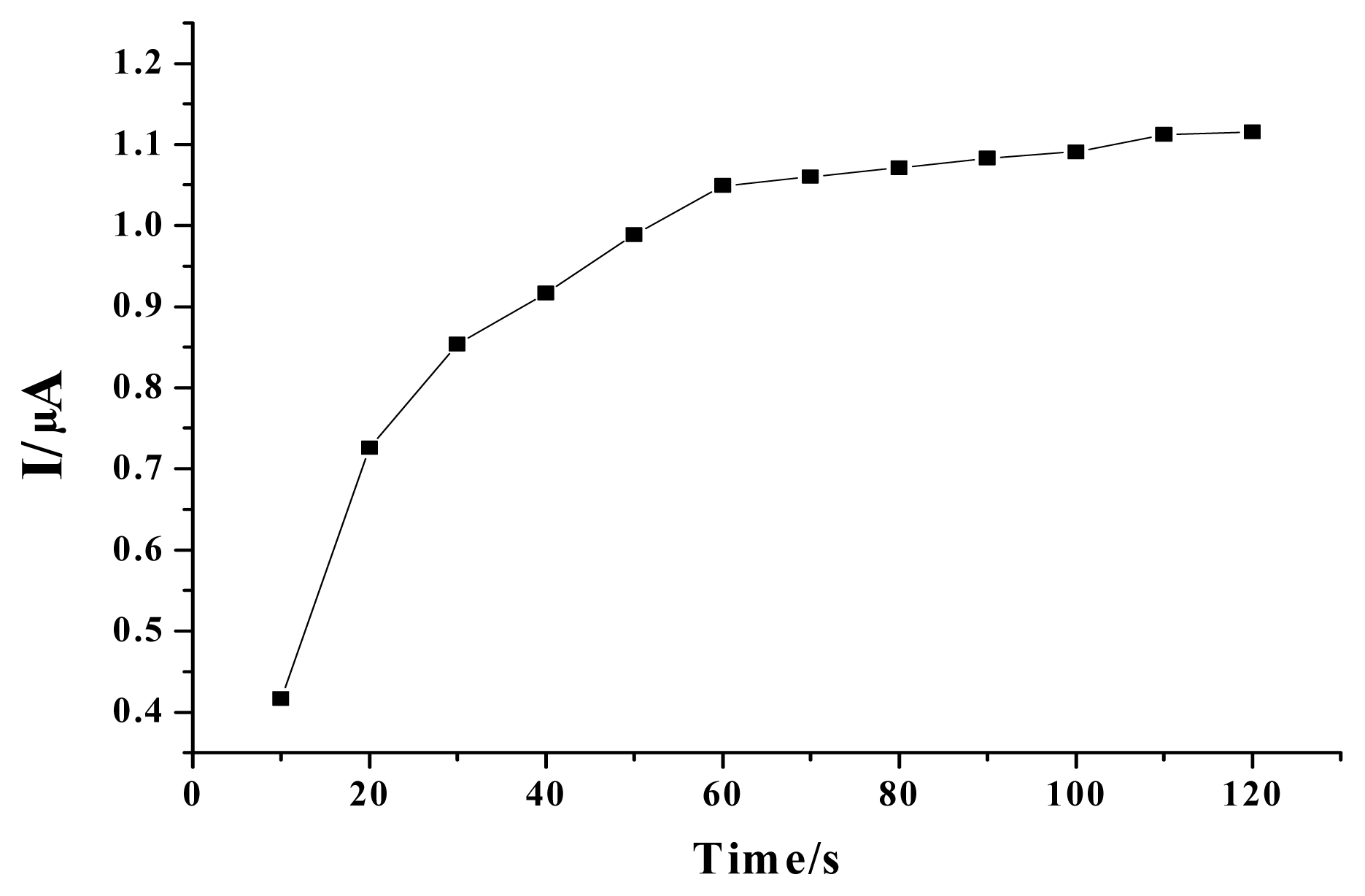
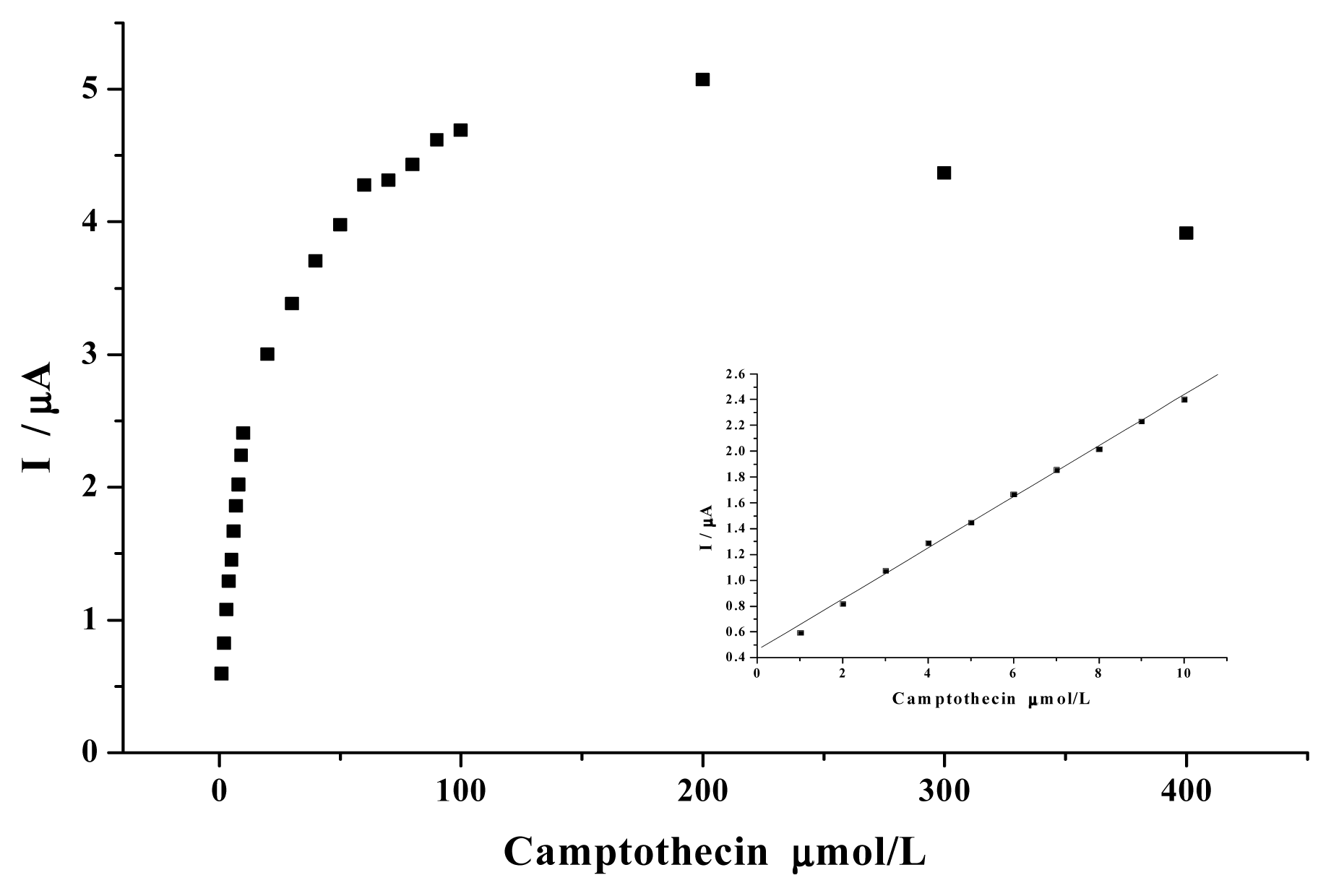
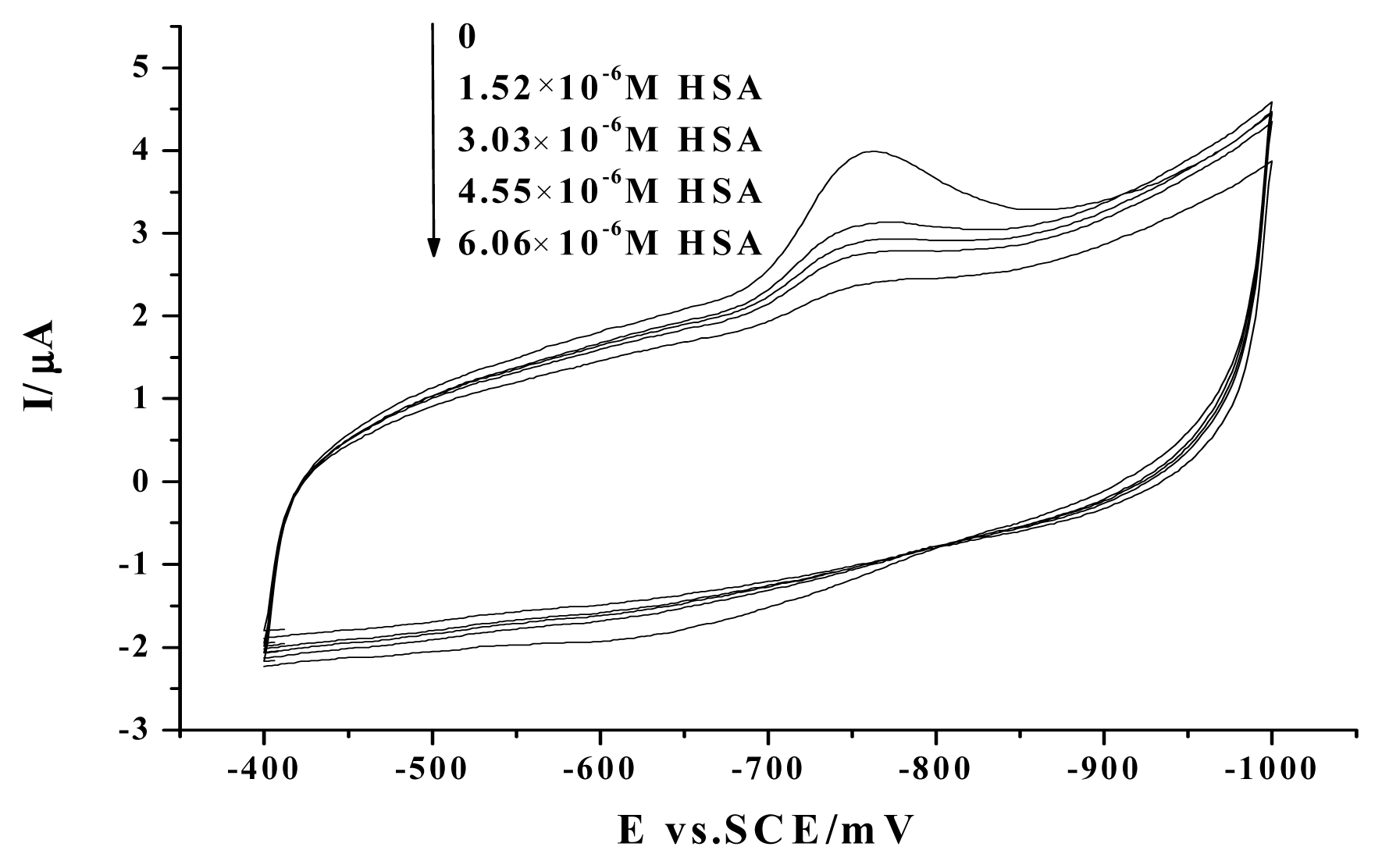
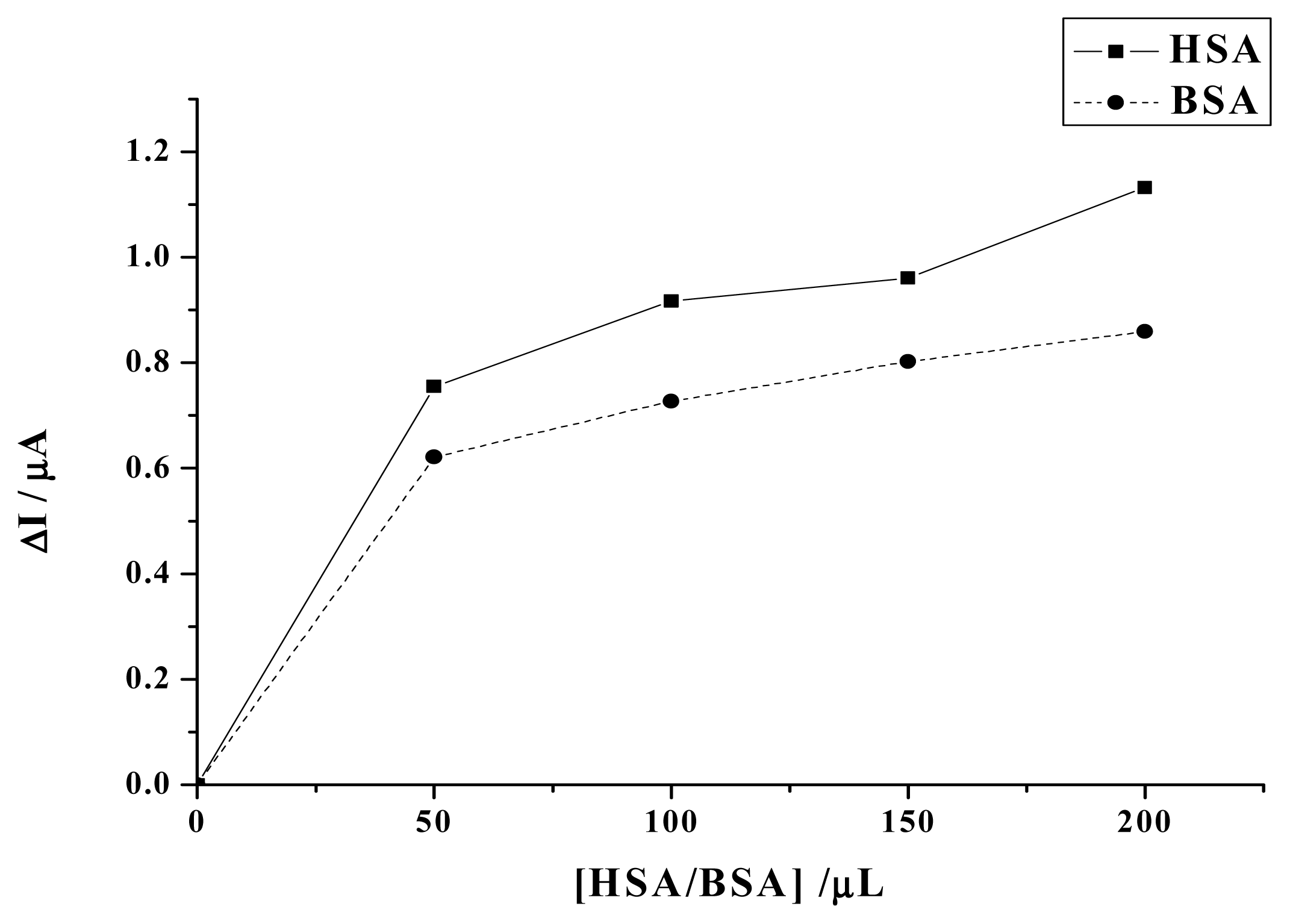
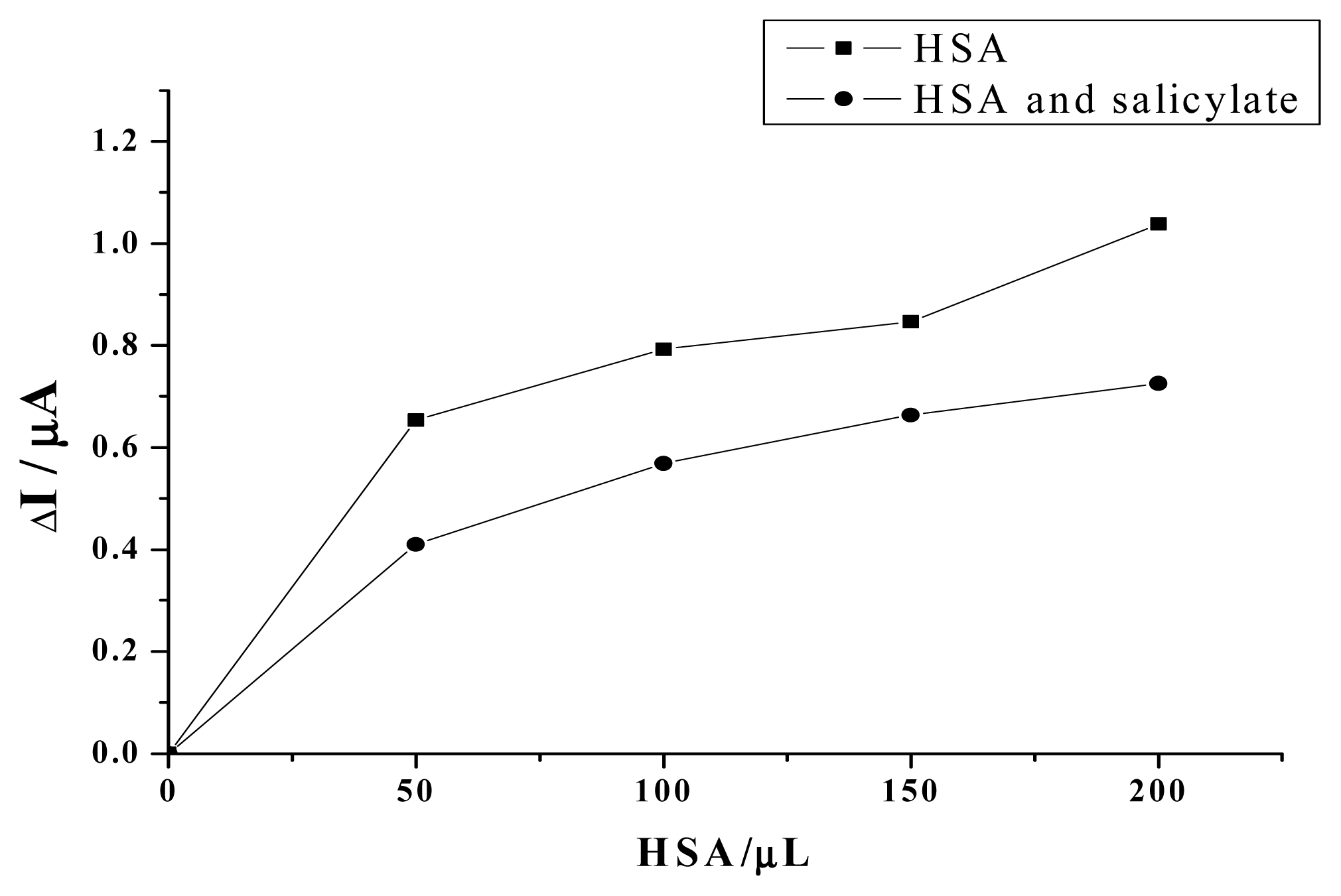
2. Results and Discussion
3. Experimental section
3. 1 Reagents
3.2 Preparation of working electrode
3.3 Apparatus
Acknowledgements
References
- Marco, E.; Laine, W.; Tardy, C.; Lansiaux, A.; Iwao, M.; Ishibashi, F.; Bailly, C.; Gago, F. Molecular Determinants of Topoisomerase I Poisoning by Lamellarins: Comparison with Camptothecin and Structure-Activity Relationships. J. Med. Chem 2005, 48, 3796–3807. [Google Scholar]
- Litvak, David A.; Papaconstantinou, Harry T.; Mark Evers, B.; Townsend, Courtney M., Jr. Targeting molecular pathways with camptothecin as novel therapy for gastric cancer. Journal of Gastrointestinal Surgery 1999, 3, 618–624. [Google Scholar]
- Liu, W.M.; Zhang, R.W. Upregulation of p21 (WAF1/CIP1) in human breast cancer cell lines MCF- 7 and MDA-MB-468 undergoing apoptosis induced by natural product anticancer drugs 10- hydroxycamptothecin and camptothecin through p53-dependent and independent pathways. International Jounal Of Oncology 1998, 12(4), 793–804. [Google Scholar]
- Sanchez-Alcazar, JA; Bradbury, DA. Brea-Calvo G, Navas P, Knox AJ Camptothecin-induced apoptosis in non-small cell lung cancer is independent of cyclooxygenase expression. Apoptosis 2003, 8(6), 639–647. [Google Scholar]
- Gao, Heyong; Zhang, Xiongwen; Chen, Yi; Shen, Hongwu; Pang, Tao; Sun, Jing; Xu, Chenghui; Ding, Jian; Li, Chuan; Lu, Wei. Synthesis and antitumor activity of the hexacyclic camptothecin derivatives. Bioorganic & Medicinal Chemistry Letters 2005, 15, 3233–3236. [Google Scholar]
- Hertzberg, R.P.; Busby, R.W.; Caranfa, M.J.; Holden, K.G.; Johnson, R.K.; Hecht, S.M.; Kingsbury, W.D. Irreversible trapping of the DNA-topoisomerase I covalent complex. Affinity labeling of the camptothecin binding site. J. Biol. Chem 1990, 265, 19287–19295. [Google Scholar]
- Chourpa, Ignor; Beljebbar, Abdel; Sockalingum, Ganesh D; Manfait, Michel. Structure–activity relation in camptothecin antitumor drugs: why a detailed molecular characterisation of their lactone and carboxylate forms by Raman and SERS spectroscopies? Biochimica et Biophyscia Acta (BBA ) -general subjects 1997, 1334, 349–360. [Google Scholar]
- Priel, E.; Showalter, S. D.; Blair, D.G. Inhibition of human. immnnodeficiency virus (HIV-I) replication by non cytotoxic. doses of camptothecin, a topoisomerase I inhibitor. AIDS Res. Hum. Retrovir 1991, 7, 65–72. [Google Scholar]
- Bodley, A.L.; Shapiro, T.A. Molecular and cytotoxic effects of camptothecin, a topoisomerase I inhibitor on trypanosomes and Leishmania. Proc. Natl. Acad. Sci 1995, 92, 3726–3730. [Google Scholar]
- Jaxel, C.; Kohn, K. W.; Wani, M. C.; Wall, M. E. Structure activity study of the actions of camptothecin derivatives on mammalian topoisomerase 1: evidence for a specific receptor site and a relation to antitumor activity. Cancer Res 1989, 49, 1465–1469. [Google Scholar]
- Roller, Shane G.; Dieckhaus, Christine M.; Santos, Webster L.; Duane Sofia, R.; Macdonald, Timothy L. Interaction between Human Serum Albumin and the Felbamate Metabolites 4- Hydroxy-5-phenyl-[1, 3]oxazinan-2-one and 2-Phenylpropenal. Chem. Res. Toxicol 2002, 15, 815–824. [Google Scholar]
- Fleury, Fabrice; Kudelina, Irina; Nabiev, Igor. Interactions of lactone, carboxylate and self-aggregated forms of camptothecin with human and bovine serum albumins. FEBS letter 1997, 406, 151–156. [Google Scholar]
- Zheng, J.B; Meng, Z.C; Liu, B.; Zhang, H.F. Investigation on voltammetric behavior of camptothecin and its analytical application. Chinese Journal of Chemistry 2006, 24, 551–556. [Google Scholar]
- Ong, E. S.; Apandi, S. N. Determination of berberine and strychnine in medicinal plants and herbal preparations by pressurized liquid extraction with capillary zone electrophoresis. Electrophoresis 2001, 22, 2723–2729. [Google Scholar]
- Laviron, E. Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J Electroanal Chem 1974, 52, 355–393. [Google Scholar]
- Nassar, A.E.F.; Zhang, Z.; Hu, N.F.; Rusling, J.F.; Kumosinski, T.T. Proton-Coupled Electron Transfer from Electrodes to Myoglobin in Ordered Biomembrane-like Films. J. Phys. Chen. B 1997, 101, 2224–2231. [Google Scholar]
- Muzammil, Salman; Kumar, Yogesh; Tayyab, Saad. Molten globule-like state of human serum albumin at low pH. Eur J. Biochem 1999, 266, 26–32. [Google Scholar]
- Li, L. Y.; Zhang, D.Y.; Bai, F.W. Research on Camptothecine (CPT) and its Derivative. Journal of Dalian Nationalities University 2001, 3, 17–22. [Google Scholar]
- Liu, W. Z.; Wang, Z.F. Accumulation and Localization of Camptothecin in Young Shoot of Camptotheca acuminate. Acta Photophysiologica Sinica 2004, 30, 405–412. [Google Scholar]
- Zhang, Z.X.; Mao, G. X.; Zhang, M.X.; Zhang, W. J. Extracting New Technique for Camptothecine by NaOH aq. Natural Product Research and Development 2006, 18, 302–303. [Google Scholar]
- Yan, X.F.; Wang, Y.; Yu, T.; Zhang, Y. H.; Yin, L.J. Determination of Camptothecin in Leaves of Camptotheca Acuminata Decne. by HPLC. Journal of Instrumental Analysis 2002, 21, 15–17. [Google Scholar]
- Hirayama, K.; Akashi, S.; Furuya, M.; Fukuhara, K. I. Rapid confirmation and revision of the primary structure of bovine serum albumin by ESIMS and Frit-FAB LC/MS. Biochem. Biophys. Res. Commun 1990, 173, 639–646. [Google Scholar]
- Inci Özer and Özden Tacal Method Dependence of Apparent Stoichiometry in the Binding of Salicylate Ion to Human Serum Albumin: A Comparison between Equilibrium Dialysis and Fluorescence Titration. Analytical Biochemistry 2001, 294, 1–6.

© 2007 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Zhao, J.; Zheng, X.; Xing, W.; Huang, J.; Li, G. Electrochemical Studies of Camptothecin and Its Interaction with Human Serum Albumin. Int. J. Mol. Sci. 2007, 8, 42-50. https://doi.org/10.3390/i8010042
Zhao J, Zheng X, Xing W, Huang J, Li G. Electrochemical Studies of Camptothecin and Its Interaction with Human Serum Albumin. International Journal of Molecular Sciences. 2007; 8(1):42-50. https://doi.org/10.3390/i8010042
Chicago/Turabian StyleZhao, Jing, Xiaofeng Zheng, Wei Xing, Junyi Huang, and Genxi Li. 2007. "Electrochemical Studies of Camptothecin and Its Interaction with Human Serum Albumin" International Journal of Molecular Sciences 8, no. 1: 42-50. https://doi.org/10.3390/i8010042
APA StyleZhao, J., Zheng, X., Xing, W., Huang, J., & Li, G. (2007). Electrochemical Studies of Camptothecin and Its Interaction with Human Serum Albumin. International Journal of Molecular Sciences, 8(1), 42-50. https://doi.org/10.3390/i8010042



