The Gibbs free energy, enthalpy and entropy of partitioning of each of the 45 chemicals in the water-octanol and water-cyclohexane systems are given in
Table 1. All of the van’t Hoff plots of log P
vs. 1/T for water-cyclohexane, and most of those for water-octanol, were rectilinear. For six compounds with non-rectilinear plots (see Experimental), tangents were taken at 25
oC. It is accepted that for these compounds the thermodynamic parameters probably contain greater error. We believe, however, that the consistency of the results means that they are reasonably accurate.
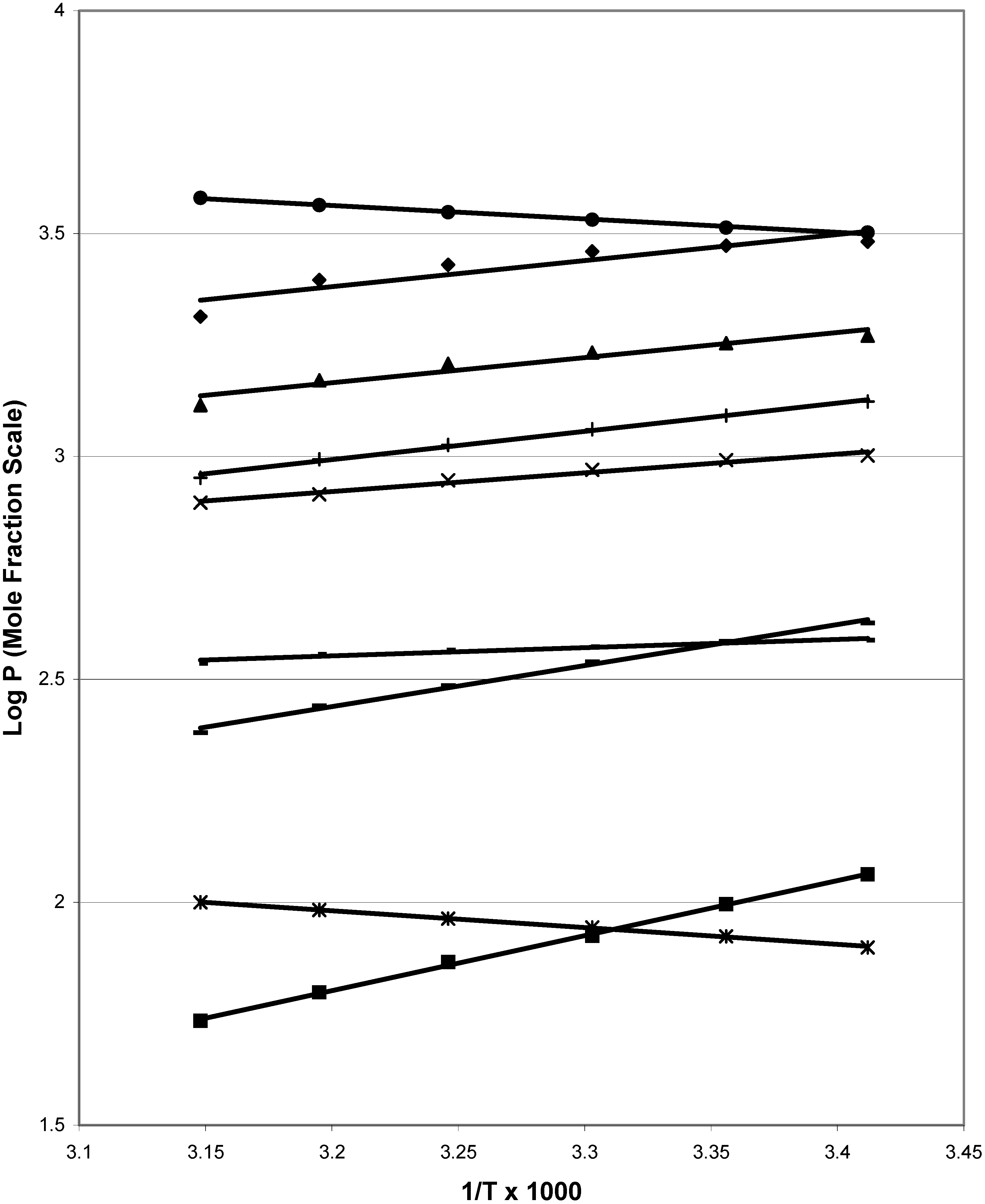
Figure 1 depicts van’t Hoff plots for nine of the chemicals, including some with non-rectilinear plots.
It has been postulated [
3] that ΔH and ΔS should be rectilinearly correlated for a system or process operating by a single mechanism, although it has been pointed out [
4] that since ΔH and ΔS are derived from the same set of data, such enthalpy-entropy compensation could be a statistical artefact. The use of ΔG-ΔH is preferred [
2] for this reason. In fact, for our data, there is good enthalpy-entropy correlation in the water-octanol system (n = 45, r
2 = 0.852) but not in the water-cyclohexane system (n = 42, r
2 = 0.529. There is virtually no ΔG-ΔH correlation in either system; however, when sub-sets of compounds are examined separately, there are good ΔG-ΔH correlations in both systems. For example, for the four methyl-2-nitrophenols in the water-octanol system the coefficient of variation (r
2) is 0.865. We interpret this to mean that our data are valid, and that there are multiple mechanisms of partitioning within our data-set, with distinctions being made even between, for example, methylphenols and dimethylphenols.
Intramolecular Hydrogen Bonding
2-Chlorophenol, 2-nitrophenol and the four methyl-2-nitrophenols, 2-nitroresorcinol, 2-hydroxy-benzaldehyde, 2-hydroxybenzoic acid (salicylic acid) and 2,6-dihydroxybenzoic acid are all capable of intramolecular hydrogen bonding. This reduces polarity and intermolecular hydrogen bonding ability, and might therefore be expected to increase partition coefficient. However, there is no consistent effect of intramolecular hydrogen bonding on octanol-water partition coefficients; 2-hydroxybenzaldehyde is more hydrophobic (ΔG more negative) than are its 3- and 4-isomers, whereas 2-nitrophenol is less hydrophobic than are its isomers.
Figure 1.
van’t Hoff plots for nine of the compounds used in this work: (●) 6-methyl-2-nitrophenol; (϶)2,3,6-trimethylphenol; (▲) 4-chlorophenol; (+) 3-methyl-2-nitrophenol; (x) 2-chlorophenol; (-) 2-hydroxybenzaldehyde; (−) 2,6-dihydroxybenzoic acid; (*) 2,6-dimethylacetanilide;(■) 3,5-dihydroxybenzoic acid.
Figure 1.
van’t Hoff plots for nine of the compounds used in this work: (●) 6-methyl-2-nitrophenol; (϶)2,3,6-trimethylphenol; (▲) 4-chlorophenol; (+) 3-methyl-2-nitrophenol; (x) 2-chlorophenol; (-) 2-hydroxybenzaldehyde; (−) 2,6-dihydroxybenzoic acid; (*) 2,6-dimethylacetanilide;(■) 3,5-dihydroxybenzoic acid.
The hydrogen bond donor ability of water is much greater than is that of octanol, whilst their hydrogen bond acceptor abilities are about the same [
5]. The involvement of the carbonyl oxygen of 2-hydroxybenzaldehyde in intramolecular hydrogen bonding thus reduces its interaction with water more than it reduces its interaction with octanol, thereby increasing hydrophobicity. The involvement of the nitro group of 2-nitrophenol in intramolecular hydrogen bonding still leaves one nitro group oxygen atom available for intermolecular hydrogen bonding with water, thus preventing an increase in hydrophobicity [
6]. The situation with salicylic acid is more complex; intramolecular hydrogen bonding leaves a carbonyl oxygen and a hydroxyl group still available for intermolecular hydrogen bonding, and it is difficult to predict the overall effect on hydrophobicity. In fact, salicylic acid is more hydrophobic (in the water-octanol system) than are its 3- and 4-isomers. This is confirmed by its low aqueous solubility (0.18% w/v at 20
oC) relative to that of its 3-isomer (0.92% w/v at 20
oC) [
7]. Theoretical studies have also shown that salicylic acid is intramolecularly hydrogen bonded in aqueous solution [
8]. The rather lower hydrophobicity of 2,6-dihydroxybenzoic acid suggests that this compound may possess only one intramolecular hydrogen bond in the water-octanol system. The similarity of the hydrophobicities and aqueous solubilities [
7] of all the chlorophenols suggests that 2-chlorophenol may not be intramolecularly hydrogen bonded in water or octanol. It has also been shown [
9] that the intramolecular hydrogen bond in 2-chlorophenol is broken in the presence of the hydrogen bond acceptor ethyl propionate.
In the water-octanol system, the enthalpy and entropy of transfer of 2-chlorophenol, 2-nitrophenol, 2-hydroxybenzaldehyde and 2-hydroxybenzoic acid are in each case less negative than are those of the corresponding 3- and 4-isomers. It is probably better to compare values between the 2- and 4-isomers (
Table 2), since the 3-isomers are not capable of classical resonance interaction between substituents.
Table 2.
Differences between 2- and 4-substituted phenols of enthalpies (ΔΔH) and entropies (ΔΔS) of transfer from water to octanol and from water to cyclohexane
Table 2.
Differences between 2- and 4-substituted phenols of enthalpies (ΔΔH) and entropies (ΔΔS) of transfer from water to octanol and from water to cyclohexane
| Compound | ΔΔHw→oa | ΔΔSw→ob | ΔΔHw→ca | ΔΔSw→cb |
|---|
| Chlorophenol | 1.9 | 2.0 | -5.4 | 3.0 |
| Nitrophenol | 16.7 | 33.0 | -17.3 | 5.5 |
| Hydroxybenzaldehyde | 5.9 | 24.7 | -18.8 | 6.8 |
| Hydroxybenzoic acid | 5.1 | 3.0 | c | c |
The positive ΔΔH values for transfer from water to octanol are a consequence of the weak hydrogen bond donor ability of octanol relative to that of water; since an intramolecularly hydrogen bonded –OH group is unavailable for intermolecular hydrogen bond donation to solvent, there is more solute-solvent interaction in water than in octanol, for intramolecularly hydrogen bonded compounds. Hence transfer from water to octanol is enthalpically less favourable. The positive ΔΔS values for transfer from water to octanol confirm the above, since less solute-solvent interaction in octanol than in water gives rise to greater disorder of solvent molecules consequent upon solute transfer. The negative ΔΔH values for transfer from water to cyclohexane indicate greater interaction of intramolecularly hydrogen bonded compounds with the non-polar solvent, probably reflecting the less polar nature of these compounds relative to their 4-isomers. This greater interaction accounts also for their low ΔΔS values relative to those for transfer from water to octanol.
It can be seen from
Table 2 that the ΔΔH and ΔΔS values for transfer of 2-chlorophenol from water to octanol are close to zero. We interpret this to indicate that the intramolecular hydrogen bond in 2-chlorophenol is very weak or non-existent in water and octanol, as already suggested by consideration of hydrophobicities. However, the negative ΔΔH value for transfer from water to cyclohexane indicates at least partial intramolecular hydrogen bonding of 2-chlorophenol in cyclohexane, leading to greater interaction with the non-polar solvent. In support of this, there is infrared spectroscopic evidence of such hydrogen bonding of 2-chlorophenol in tetrachloromethane [
10]. Entropically, the increased interaction with cyclohexane is more than offset by the release of structured water upon transfer from the aqueous phase.
The strongly positive ΔΔH
w→o and ΔΔS
w→o values for transfer of 2-nitrophenol indicate that the intramolecular hydrogen bond is intact in this compound in both solvents, as found also by Abraham
et al. [
6]. The significantly negative ΔΔH
w→c value, indicating strong solute-cyclohexane interaction, also confirms the existence of the intramolecular hydrogen bond of 2-nitrophenol in cyclohexane solution. As with 2-chlorophenol, the effect of such increased interaction is more than offset by the release of structured water upon transfer from the aqueous phase, leading to a slightly positive ΔΔS
w→c value.
Similar interpretations can be placed on the thermodynamic parameters for phase transfer of 2-hydroxybenzaldehyde. The low ΔΔH
w→o value for this compound may indicate that its intramolecular hydrogen bond is not wholly intact in polar solvents, in agreement with the findings of Berthelot
et al. [
11]. The ΔΔH
w→c and ΔΔS
w→c values, however, confirm that it is intact in cyclohexane solution.
The ΔΔHw→o and ΔΔSw→o values for transfer of 2-hydroxybenzoic acid are, like those of 2-chloro-phenol, quite close to zero. However, as pointed out above, aqueous solubility values indicate that salicylic acid is intramolecularly hydrogen-bonded in water, and therefore presumably also in octanol. The low ΔΔHw→o and ΔΔSw→o values for 2-hydroxybenzoic acid are probably due to the fact that the compound still has an –OH group able to form intermolecular hydrogen bonds with both water and octanol.
Both 2-nitroresorcinol and 2,6-dihydroxybenzoic acid are theoretically capable of forming two intramolecular hydrogen bonds. The increase in hydrophobicity in the water-octanol system of 2-nitro-resorcinol relative to that of 2-nitrophenol implies that the compound does possess two intramolecular hydrogen bonds. However, the lower ΔH
w→o value of the former suggests that only one intramolecular hydrogen bond exists in 2-nitroresorcinol in water and octanol solutions, as does the close similarity of the ΔS
w→o values of 2-nitrophenol and 2-nitroresorcinol. Berthelot
et al. [
12] have shown that compounds with the potential to form two intramolecular hydrogen bonds actually form only one in the presence of a polar hydrogen bond donor.
The situation appears different for 2-nitroresorcinol in cyclohexane solution. Its ΔH
w→c value is more negative than is that of 2-nitrophenol, which suggests that the compound possesses two intramolecular hydrogen bonds in cyclohexane solution. Its relatively low ΔS
w→c value confirms this, since it suggests that there is relatively little structured water around 2-nitroresorcinol molecules in aqueous solution, leading to a relatively small entropy increase upon transfer to cyclohexane. Boykin [
13] has observed both intramolecular hydrogen bonds of 2-nitroresorcinol to be intact in toluene solution. Ultraviolet spectroscopic data support the above hypothesis. In contrast to the behaviour of 2-nitrophenol, we found a pronounced hypsochromic shift of the spectrum of 2-nitroresorcinol on going from cyclohexane to ethanolic or aqueous solution, consistent with breaking of an intramolecular hydrogen bond and consequent loss of planarity of the nitro group.
The lower hydrophobicity of 2,6-dihydroxybenzoic acid relative to that of salicylic acid suggests that this compound also has only one intramolecular hydrogen bond in water and octanol. Its entropy of transfer from water to octanol is considerably lower than that of salicylic acid, suggesting hydrogen bond donation to octanol. The enthalpies of transfer of the two compounds are the same, arising presumably from the very similar hydrogen bond acceptor abilities of water and octanol. That there is certainly one intramolecular hydrogen bond present in 2,6-dihydroxybenzoic acid is shown by the compound’s greater hydrophobicity and less negative ΔHw→o and ΔSw→o values relative to 3,5-dihydroxybenzoic acid. The water-cyclohexane data also indicate that, unlike the situation in 2-nitroresorcinol, there is only one intramolecular hydrogen bond present in 2,6-dihydroxybenzoic acid in cyclohexane solution. This follows because the changes in ΔGw→c, ΔHw→c and ΔSw→c relative to those for salicylic acid all mirror those for transfer from water to octanol.
Steric effects
In the compounds that we have examined, two main steric effects may be postulated: shielding of a polar group, and twisting of a polar group out of the plane of the aromatic ring, by adjacent alkyl substitution. The ΔGw→o, ΔHw→o and ΔSw→o values of the three cresols (methylphenols) are all very similar, suggesting that there is no steric shielding of the –OH group in 2-methylphenol. For transfer from water to cyclohexane a slight effect could be apparent, although, bearing in mind the experimental errors in the determination of thermodynamic parameters by the van’t Hoff method, this could be an artefact. If the effect is real, our interpretation is that shielding of the –OH group renders 2-methyl-phenol less polar, thereby increasing the partition coefficient and lowering ΔGw→c. However, this is not confirmed by the ΔHw→c and ΔSw→c values, so our conclusion must be that the data show no consistent indication of steric shielding in 2-methylphenol.
2,6-Xylenol (2,6-dimethylphenol) does, however, show a significant shielding effect. Transfer from water to octanol involves a less negative ΔHw→o value and a more positive ΔSw→o value than those of the other isomers; its ΔGw→o value, however, is very close to those of its isomers. The data are interpreted as indicating more structured water around the molecule in aqueous solution, leading to breaking of hydrogen bonds between water molecules upon transfer to octanol and a consequent increase in disorder. Transfer from water to cyclohexane involves a more negative ΔGw→c value and less positive ΔHw→c and ΔSw→c values than those of the other isomers. These all indicate a significant solute-cyclohexane interaction, consistent with appreciable shielding of the –OH group and lowering of effective polarity.
Additional methyl substitution does not appear to give rise to additional shielding, even in 2,3,6-trimethylphenol and 2,3,5,6-tetramethylphenol, where a buttressing effect of two adjacent methyl groups might have been expected. The methyl-substituted benzoic acids show a somewhat similar pattern to the methyl-substituted phenols. Despite the greater size of the carboxyl group compared with the hydroxyl group, there is no evidence of any steric shielding or twisting in 2-methylbenzoic acid in the water-octanol system, since the thermodynamic parameters for all three isomers are very similar. There is some evidence of steric shielding from the water-cyclohexane data, with ΔH
ow→c being less positive, and ΔG
w→c being slightly more negative, for 2-methylbenzoic acid compared with the 4-isomer, as is the case for the xylenols. However, the low ΔS
w→c value for 2-methylbenzoic acid is not in line with dimethylphenol behaviour, and could indicate some twisting of the carboxyl group, thereby reducing the extent of water-structuring in the aqueous phase. Cisarova
et al. [
14] have shown by X-ray diffraction that the carboxyl group of 2,3-dimethylbenzoic acid is non-planar. The data for 3-methylbenzoic acid appear to be anomalous, and we have no explanation for this, other than that the data are erroneous.
2,6-Dimethylbenzoic acid, however, shows clear evidence of steric twisting of the carboxyl group, leading to loss of conjugation of the group with the aromatic ring. This renders the group more hydrophilic [
15], and hence both ΔG
w→o and ΔG
w→c are less negative than the corresponding values for 3,5-dimethylbenzoic acid. Similarly, ΔH
w→o and ΔH
w→c are both less negative than those of 3,5-dimethylbenzoic acid, which may be attributed to strong interaction of the hydrophilic non-planar carboxyl group of 2,6-dimethylbenzoic acid with water. There is little effect of non-planarity of the carboxyl group on ΔS
w→o. The ΔS
w→c value of 3,5-dimethylbenzoic acid is anomalously low, which may be attributable to self-association of this compound in cyclohexane; benzoic acids are known to undergo self-association at very low concentrations [
16]. 2,6-Dimethylbenzoic acid would not be expected to self-associate because its carboxyl group is shielded.
Dearden and O’Hara [
17] drew attention to the unusual partitioning behaviour of 2-methyl-acetanilide in the water-octanol system, and attributed it to loss of planarity of the acetamido group. It can be seen from
Table 1 that the thermodynamic data confirm this, as ΔG
w→o and ΔG
w→c are less negative than the corresponding values for both the 3- and 4-isomers, indicating the greater hydrophilicity of the non-planar acetamido group. ΔH
w→o is less negative (more positive), which probably indicates a reduced ability of octanol to interact with a shielded acetamido group; the high ΔS
w→o value confirms this, being indicative of greater disorder in octanol than in aqueous solution. ΔH
w→c, however, is less positive, suggesting a partial screening of polarity and thus greater interaction with cyclohexane. Again, the entropy data confirm this, with ΔS
w→c indicating a lower increase in disorder on water-cyclohexane transfer than for the 3- and 4-isomers. The data for 2,6-dimethyl-acetanilide, relative to its 3,5-isomer, are entirely consistent with the above explanations.
A very interesting aspect of steric effects is shown by the methyl-2-nitrophenols. Dearden and Forbes [
18] used ultraviolet absorption spectroscopy to show that in 3-methyl-2-nitrophenol steric hindrance by the methyl group puts a strain upon the intramolecular hydrogen bond. In cyclohexane solution this strain is insufficient fully to rupture the hydrogen bond, as was observed by Boykin [
13] for the compound in toluene solution. However, in hydrogen bonding solvents such as water and ethanol, competing solute-solvent hydrogen bonding is sufficient to cause rupture of the intramolecular hydrogen bond. In 6-methyl-2-nitrophenol, on the other hand, the effect of the methyl group is to push the hydroxyl group closer to the nitro group, thus strengthening the intramolecular hydrogen bond.
The partitioning data largely support the above. ΔHw→o is much more negative for 3-methyl-2-nitrophenol than for the other isomers, suggesting less structured water present, and therefore fewer water-water hydrogen bonds to be broken on transfer to octanol; this is confirmed by the very low ΔSw→o value for 3-methyl-2-nitrophenol. Conversely, for 6-methyl-2-nitrophenol, with a very strong intramolecular hydrogen bond, ΔHw→o is actually positive and ΔSw→o is much more positive than for the other isomers. The water-cyclohexane transfer data indicate that the intramolecular hydrogen bond in 3-methyl-2-nitrophenol is intact in cyclohexane solution, as ΔHw→c values are similar in 3-, 4- and 5-methyl-2-nitrophenols. The high strength of the intramolecular hydrogen bond in 6-methyl-2-nitrophenol is shown by its less negative ΔHw→c value and its highly positive ΔSw→c value, both of which indicate a high level of structured water in aqueous solution, leading to rupture of water-water hydrogen bonds and concomitant increase in disorder upon transfer to cyclohexane.