Among Synthetic, Supramolecular and Theoretical Chemistry Stabilization of Short-lived Species in “Molecular’ or ‘Supramolecular Flasks’
Abstract
:I. Introduction
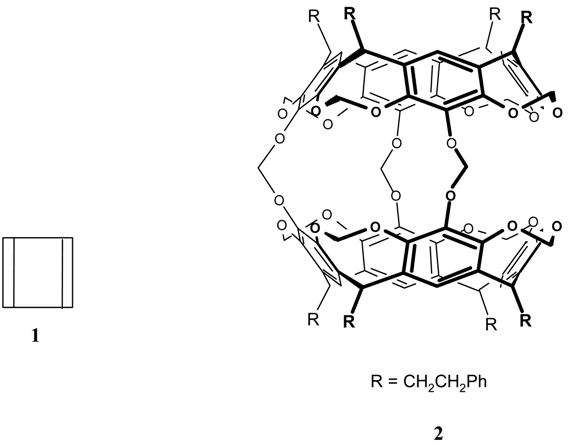
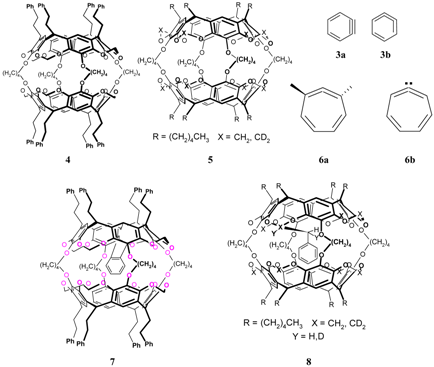
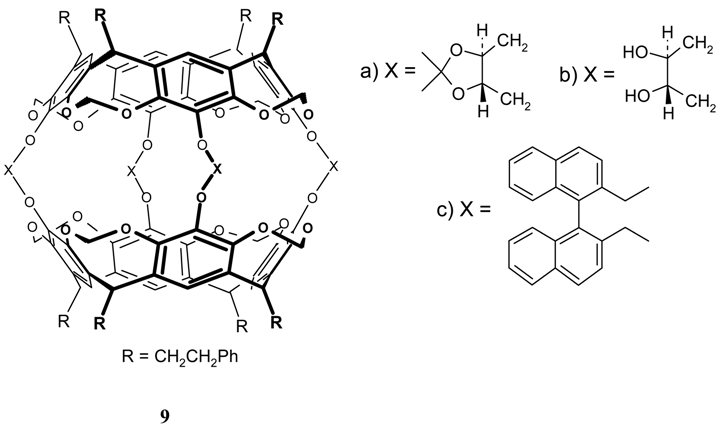
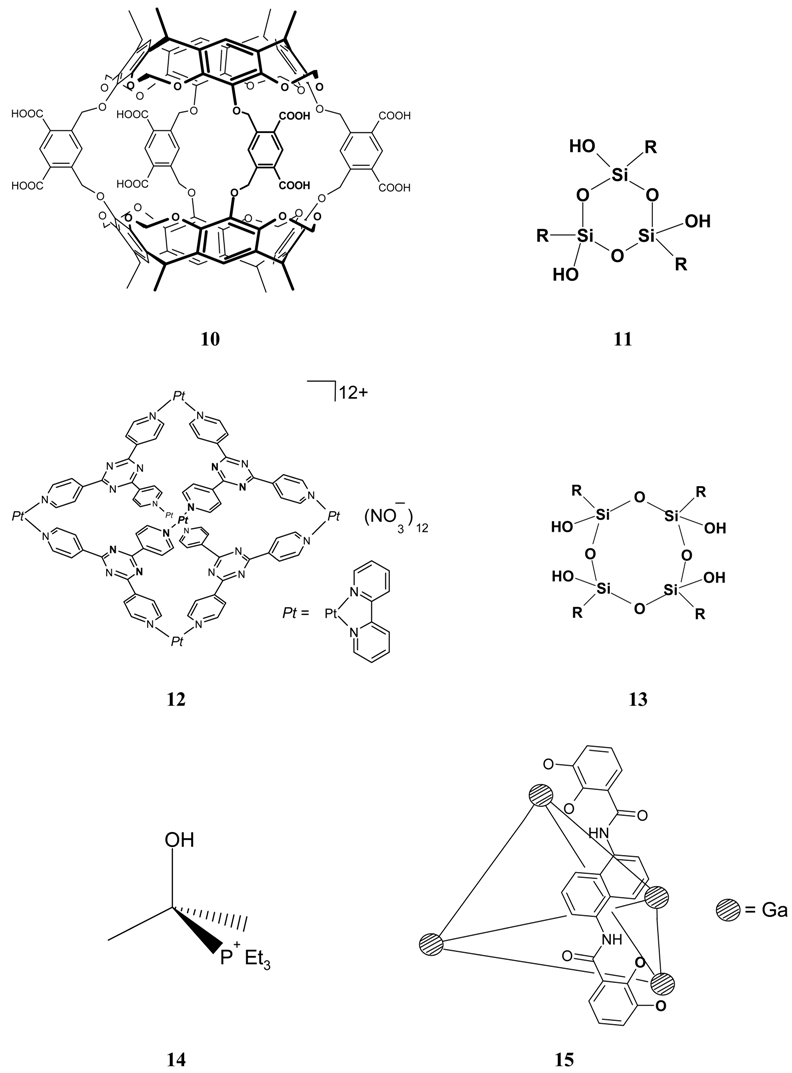
References
- Dodziuk, H. Introduction to Supramolecular Chemistry; Kluwer: Dordrecht, 2002. [Google Scholar]Lehn, J.-M. Supramolecular Chemistry. Concepts and Perspectives. VCH: Weinheim, 1995. [Google Scholar]Comprehensive Supramolecular Chemistry. vol. 3, Szejtli, J. (Ed.) Elsevier: Oxford, 1996.New Trends in Cyclodextrins and Derivatives. Duchene, D. (Ed.) Editions du Sante: Paris, France, 1991.
- Weidinger, A.; Waiblinger, M.; Pietzak, B.; Murphy, T. A. Appl. Phys. A 1998, 66, 287.
- Khong, A.; Jimenez-Vazquez, H. A.; Saunders, M.; Cross, R. J.; Laskin, J.; Peres, T.; Lifshitz, C.; Strongin, B.; Smith III, A. B. J. Am. Chem. Soc. 1998, 120, 6380.Laskin, J.; Peres, T.; Lifshitz, C.; Saunders, M.; Cross, R. J.; Khong, A. Chem. Phys. Lett. 1998, 285, 7.
- Wagner, M. J.; Dye, J. L. Comprehensive Supramolecular Chemistry; Lehn, J.-M., Ed.; Elsevier: Oxford, 1996; vol. 1, p. 477. [Google Scholar]
- Tanner, M. E.; Knobler, C. B.; Cram, D. J. J. Am. Chem. Soc. 1990, 112, 1659. [CrossRef]
- Cram, D. J.; Tanner, M. E.; Thomas, R. Angew. Chem. Int. Ed. 1991, 30, 1924.
- Maier, G. Angew. Chem. Int. Ed. 1988, 27, 309. [CrossRef]
- Bally, T.; Masamune, S. Tetrahedron; 1980; Volume 36, p. 343. [Google Scholar]
- Corey, E. J.; Streigth, J. J. Am. Chem. Soc. 1964, 86, 950. [CrossRef]Corey, E. J.; Pirkle, W. H. Tetrahedron Lett. 1967, 5255.
- Hopf, H. Angew. Chem. Int. Ed. 1991, 30, 1117.
- Deniz, A. A.; Peters, K. S.; Snyder, G. J. Science 1999, 286, 1119.
- Sancho-Garcia, J. C.; Pittner, J.; Carsky, P.; et al. J. Chem. Phys. 2000, 112, 8785. [CrossRef]
- Metropoulos, A.; Chiu, Y. N. J. Mol. Struct. (THEOCHEM) 1997, 417, 95. [CrossRef]
- Jemmis, E. D.; Subramanian, G.; Korkin, A. A.; et al. J. Phys. Chem. A 1997, 101, 919. [CrossRef]Petersson, E. J.; Fanuele, J. C.; Nimlos, M. R.; et al. J. Am. Chem. Soc. 1997, 119, 11122. [CrossRef]
- Warmuth, R. Angew. Chem. Int. Ed. 1997, 36, 1347–1350.
- Warmuth, R. Chem. Commun. 1998, 59.Beno, B. R.; Sheu, C.; Houk, K. N.; Warmuth, R.; Cram, D. J. Chem. Commun. 1998, 301.
- Radziszewski, J. G.; Hess, B. A., Jr.; Zahradnik, R. J. Am. Chem. Soc. 1992, 114, 52.Simon, J. G. G.; Münzel, N.; Schweig, A. Chem. Phys. Lett. 1990, 170, 187.
- Jiao, H. J.; Schleyer, P. R.; Beno, B. R.; Houk, K. N.; Warmuth, R. Angew. Chem. Int. Ed. 1998, 36, 2761. [CrossRef]
- West, P. R.; Chapman, O. L.; LeRoux, J.-P. J. Am. Chem. Soc. 1982, 104, 1779. [CrossRef]MacMahon, R. J.; Albelt, C. J.; Chapman, O. L.; Johnson, J. W.; Kreil, C. L.; LeRoux, J.-P. J. Am. Chem. Soc. 1982, 104, 1779. [CrossRef]
- Warmuth, R.; Marvel, M. A. Angew. Chem. Int. Ed. 2000, 39, 1117.
- Park, B. S.; Knobler, C. B.; Warmuth, R.; Cram, D. J. Chem. Commun. 1998, 55.
- Yoon, J. Y.; Cram, D. J. Chem. Commun. 1997, 497.
- Yoshizawa, M.; Kusakawa, T.; Fujita, M.; Yamaguchi, K. J. Am. Chem. Soc. 2000, 122, 6311. [CrossRef]
- Baney, R. H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Chem. Rev. 1995, 95, 1409.
- Ziegler, M.; Brumaghim, J. L.; Raymond, K. N. Angew. Chem. Int. Ed. Engl. 2000, 39, 4119.
- Dodziuk, H. Modern Conformational Analysis. Elucidating Novel Exciting Molecular Structure; VCH Publishers: New York, 1995; Chapters 7, 8. [Google Scholar]
- Zefirov, I. S.; Koz’min, A. S.; Abramenkov, A. B. Usp. Khim. 1978, 47, 289, Engl. transl. p. 163.Maier, G.; Pfriem, S.; Schäfer, U.; Matusch, R. Angew. Chem. Int. Ed. Engl. 1978, 17, 520. [CrossRef]Maier, G.; Born, D. Angew. Chem. Int. Ed. Engl. 1989, 28, 1050. [CrossRef]
- Brousseau, R. J. Ph. D. thesis, Harvard University. Diss. Abstr. Int. 1977, B37, 6123b. [Google Scholar]Disch, R. L.; Schulman, J. M. J. Am. Chem. Soc. 1990, 112, 3377. [CrossRef]
- Dodziuk, H. J. Mol. Struct. 1990, 239, 167. [CrossRef]
- Rasmussen, D. R.; Radom, L. Angew. Chem, Int. Ed. Engl. 1999, 38, 2876.
- Dodziuk, H.; Leszczynski, J.; Nowinski, K. S. J. Org. Chem. 1995, 60, 6860. [CrossRef]
- Dodziuk, H.; Leszczynski, J.; Jackowski, K. J. Org. Chem. 1999, 64, 6177. [CrossRef]
- Seidl, E. T.; Schäfer III, H. F. J. Am. Chem. Soc. 1991, 113, 1915. [CrossRef]
- Maier, G.; Pacl, H.; Reisenauer, H. P.; Meudt, M.; Janoschek, R. J. Am. Chem. Soc. 1995, 117, 12712. [CrossRef]
- Prinzbach, H.; Weller, A.; Landenberger, P.; Wahl, F.; Worth, J.; Scott, L. T.; Gelmont, M.; Olevano, D.; von Issendorff, B. Nature 2000, 407, 60.
- McDowell, S. A. C. J. Chem. Phys. 2001, 114, 8395.
- Bourissou, D.; Guerret, O.; Gabbai, F. P.; Bertrand, G. Chem. Rev. 2000, 100, 39.
© 2002 by MDPI (http://www.mdpi.org).
Share and Cite
Dodziuk, H. Among Synthetic, Supramolecular and Theoretical Chemistry Stabilization of Short-lived Species in “Molecular’ or ‘Supramolecular Flasks’. Int. J. Mol. Sci. 2002, 3, 814-821. https://doi.org/10.3390/i3070814
Dodziuk H. Among Synthetic, Supramolecular and Theoretical Chemistry Stabilization of Short-lived Species in “Molecular’ or ‘Supramolecular Flasks’. International Journal of Molecular Sciences. 2002; 3(7):814-821. https://doi.org/10.3390/i3070814
Chicago/Turabian StyleDodziuk, Helena. 2002. "Among Synthetic, Supramolecular and Theoretical Chemistry Stabilization of Short-lived Species in “Molecular’ or ‘Supramolecular Flasks’" International Journal of Molecular Sciences 3, no. 7: 814-821. https://doi.org/10.3390/i3070814
APA StyleDodziuk, H. (2002). Among Synthetic, Supramolecular and Theoretical Chemistry Stabilization of Short-lived Species in “Molecular’ or ‘Supramolecular Flasks’. International Journal of Molecular Sciences, 3(7), 814-821. https://doi.org/10.3390/i3070814



