Abstract
The hydrogen bonded and van der Waals isomers of pyrrole···nitrogen and pyrrole···carbon monoxide have been studied using ab initio and density functional theory methods. Complex geometries and total energies of the isomers have been determined at HF, MP2, B3LYP and B3PW91 levels of theory employing 6-31G* basis set. For pyrrole···nitrogen complex, only two isomers have stable structure and the more stable one is found to be the hydrogen bonded isomer. Among the five isomers of pyrrole···carbon monoxide complex, the hydrogen bonded isomer is found to be the most stable form. The interaction energy for all these isomers have been calculated after eliminating the basis set superposition errors by using the full counterpoise correction method. Chemical hardness, chemical potential have been calculated and are used to study the stability of the molecules.
Introduction
Generally, electromagnetic forces are of fundamental importance in chemistry and they are manifested in the formation of covalent bonds between atoms, which lead to the formation of molecules and non covalent bonds between molecules leading to the formation of intermolecular associates. The former, named as covalent interactions are connected with the formation or decay of covalent bonds, and the later, van der Waals interactions with the formation or decay of van der Waals bonds. The stabilization of bond energy accompanying the formation of a covalent bond comes from the overlap either between partially occupied orbitals or between the highest occupied molecular orbital (HOMO) of an electron donor and the lowest unoccupied molecular orbital (LUMO) of an electron acceptor. When a van der Waals bond is formed, the bonding orbitals of the interacting sub systems are completely occupied by electrons and all the antibonding orbitals are unoccupied. Interaction between completely occupied orbitals leads to destabilization of a molecule. The stabilization energy originates from the interaction between permanent multipoles, between permanent multipole and an induced multipole, or between an instantaneous multipole and an induced multipole; the respective energy terms are called Coulombic, induction, and dispersion [1].
Nitrogen and carbon monoxide molecules are isoelectronic and have electric quadrupole moment, the later molecule has an additional dipole moment, which is found to be very small. The interaction sites in the aromatic ring for the nitrogen and carbon monoxide [2] have been intensively studied for the past few years. The experimental studies on benzene…nitrogen [3] and benzene…carbon monoxide [4] indicate that the diatomic molecules bind with the benzene parallel above the center of the ring and is free to rotate around the six fold axis, CO molecule is tilted by 4.6o away from the parallel arrangement and has the carbon end nearer to the benzene plane. Ab initio calculations have predicted that the benzene…nitrogen is stable and the barrier height is found to be minimum. The studies on halogen substituted benzene nitrogen complexes have also confirmed that the nitrogen is present above the ring [5]. There are several studies on interaction of nitrogen with hydroxial group indicated that the ligand is bound quasi linear to the hydrogen of the polar solvent via a weak hydrogen bond [2,6,7,8,9]. Here, the dominant stabilization force will be the dipole-quadrupole interaction. The carbon monoxide has two possibilities to attach to water from either end (ie) carbon end or oxygen end and these isomers have been observed in noble gas matrices [10], but the molecular beam experiment confirmed the OC…H2O isomer [2,6]. A few experimental studies have also confirmed the hydrogen bonded form for both the complexes.
Generally, the measurement of the accurate dissociation energies provides the information about the van der Waals potential energy surfaces. The [phenol.CO]+ have been studied using the zero kinetic energy spectroscopy (ZEKE) and mass analyzed threshold ionization (MATI) spectroscopy to investigate the interaction of the CO ligand with a hydrogen bonding [11]. Recently, the hydrogen bonded and van der Waals isomers of phenol…nitrogen and phenol…carbon monoxide in their neutral electronic (S0) and cation ground state (D0) for the several possible structures have been studied using HF/6-31G*, MP2/6-31G* and B3LYP/6-31G* levels of theory [2]. All levels of ab initio and DFT methods have predicted the hydrogen bonded isomer as stable one, which is in agreement with the experimental results. It is due to the reduction in the dispersion interaction up on ionization. Pyrrole is one of the important molecules in the five membered aromatic heterocycles. Limited experimental studies have been reported for the structure of this molecule [12,13,14]. But at the same time there are few reports available on the vibrational spectroscopy of this molecule [14,15,16,17]. Experimental IR and Raman spectroscopy [18], quantum chemical and density functional theory [19] studies on vibrational spectroscopy for the pyrrole molecule have been reported. Since the recent studies on phenol…nitrogen and phenol…carbon monoxide gave some interesting results, which stimulate us to study the pyrrole…nitrogen and pyrrole…carbon monoxide molecules using the ab initio and DFT methods. Moreover, we are very much interested to study the impact of nitrogen and carbon monoxide molecules on the five membered ring.
Computational details
All calculations have been carried out using Gaussian 94W program package [20] for pyrrole..nitrogen and pyrrole..carbon monoxide complexes. The geometries of these two complexes and their isomers have been optimized at HF/6-31G* and MP2/6-31G* levels of ab initio method, B3LYP/6-31G* and B3PW91/6-31G* levels of theory of DFT method. In DFT methods, Becke’s three parameter exact exchange-functional (B3) [21] combined with gradient-corrected correlational functional of Lee, Yang and Parr (LYP) [22] and Perdew and Wang (PW91) [23] were used implementing 6-31G* basis set. The density functional theory parameters such as chemical hardness (η) and chemical potential (μ) for these complexes were calculated at HF/6-31G* and MP2/6-31G* levels of theory.
The chemical potential [24] and chemical hardness [25] are defined as
where E is the total energy, v(r) is the external potential, and N is the number of electrons. In a finite difference approximation, with the assumption that the energy varies quadratic with the number of electrons, these two quantities can be expressed with an orbital basis set as
where I is the ionization potential and A is the electron affinity of a system.
One must be careful to treat the basis set superposition error (BSSE) problem, when measuring the interaction energy of the molecular complexes. The description of internal monomer properties depends on the quality and location of the basis functions of the partner molecule(s). Due to BSSE, the computed interaction energies become too high and the predicted potential energy surfaces are distorted [26]. To get the correct interaction energy, full counterpoise (CP) correction method of Boys and Bernardi [27], has been used by the following equation
where EAB(AB) is the energy of the complex with the basis set of monomers A and B, EA(AB) is the energy of monomer A with the full dimer basis set by setting the appropriate nuclear charges to zero which is located at the same intermolecular configuration as in the complex, with similar to other.
Eint(corr) = EAB(AB) - [EA(AB)+ EB(AB)]
Results and discussion
PYRROLE···NITROGEN COMPLEX
Geometries and energies
There are number of structures possible for the pyrrole…nitrogen complex. Of all the above, the only two structures are found to be reasonable in terms of energies. Nitrogen molecule can interact with some molecules, but, the N2…H2O, benzene…nitrogen and phenol…nitrogen complexes are important, which are very close to the present complex, pyrrole…nitrogen. The past studies on neutral N2…H2O complex have predicted that the hydrogen bond in the above complex is stronger than the benzene…nitrogen complex. In the recent studies on phenol…nitrogen complex, many possible complex structures (ie) the nitrogen molecule is: above the ring, perpendicular to the ring, inside the ring, T-shaped isomer and hydrogen bonded isomers have been considered. The HF, MP2 and B3LYP levels of theory with 6-31G* basis set have predicted that the hydrogen bonded isomer is found to be the global minimum among the above different isomers. In the present study also, we have tried as many as pyrrole…nitrogen isomers, but it was found that the two isomers, which are shown in Fig. 1, are more reasonable.
The geometrical parameters of these two isomers, calculated at HF/6-31G*, MP2/6-31G*, B3LYP/6-31G* and B3PW91/6-31G* levels of theory have been shown in Table 1 and the values are compared with the experimental values of isolated pyrrole molecule [28,29]. The MP2/6-31G* level of theory has predicted the geometrical parameters which are very close to the experimental microwave data. The HF/6-31G* level of theory underestimates the N-C and C2-C3 bond lengths compared to the experimental values. Further, it has been noticed that there is not much significant shortening or lengthening of bond lengths of pyrrole molecule while forming the complex molecule. However, the interaction of nitrogen molecule with pyrrole in the linear form, shortened the
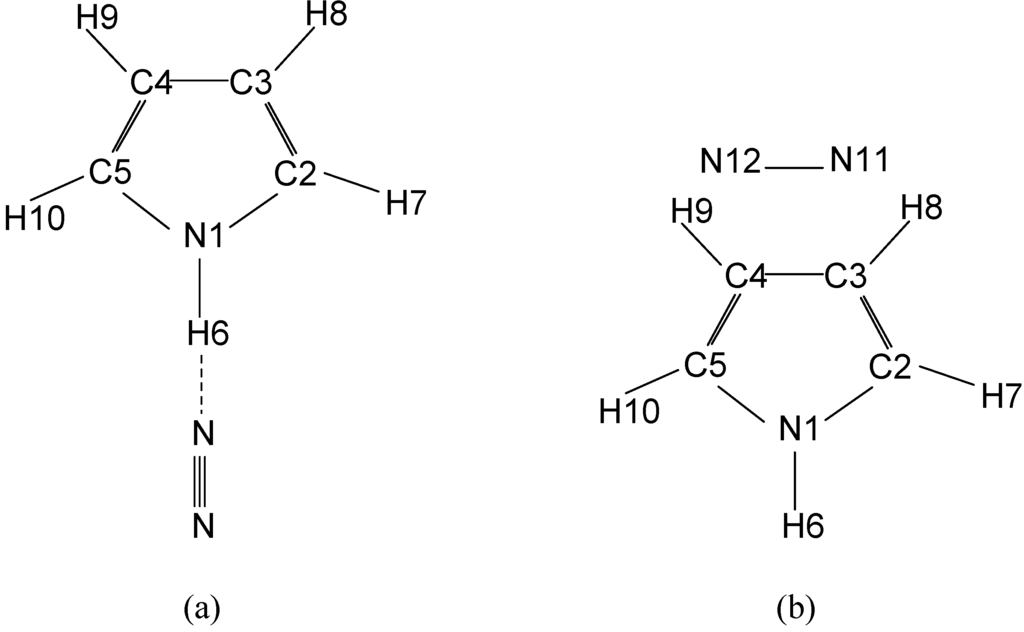

C2-H7 bond length and the average decrease in the bond length at the above four levels of theory is 0.013Å. All the above theories have predicted an average decrease in the bond angle of N1C2H7 as 5.2o. This decrease in bond angle is due to the interaction of the hydrogen bonded nitrogen with the pyrrole molecule. The hydrogen bond distance for this isomer at HF/6-31G*, MP2/6-31G*, B3LYP/6-31G* and B3PW91/6-31G* levels of theory are 2.659, 2.379, 2.431 and 2.518 Å, respectively. There is no appreciable change within the ring parameters. In this isomer the nitrogen molecule is almost linear with the N1-H6 group of pyrrole. The interaction energy and relative energy for this molecule, calculated at HF/6-31G* MP2/6-31G*, B3LYP/6-31G* and B3PW91/6-31G* levels of theory are presented in Table 2.

Figure 1.
Isomers of pyrrole…nitrogen complex (a) hydrogen bonded, (b) T – shaped isomer.

Table 1.
Optimised geometrical parameters (bond length in Å, bond angle in degrees), total energy E (in Hartree) rotational constants RA,RB,,RC (in GHz), dipole moment μM (in Debye), chemical hardness η (in eV) and chemical potential μ (in eV) for pyrrole..N2 complex.
| Parameters | Linear | T-shaped | Experimental value | |||||||||
| HF | MP2 | B3LYP | B3PW91 | HF | MP2 | B3LYP | B3PW91 | MW (Ref. 28) | MW corrected (Ref. 29) | |||
| R(N1-C2) | 1.362 | 1.373 | 1.375 | 1.370 | 1.363 | 1.373 | 1.376 | 1.371 | 1.370 | 1.370 | ||
| R(C2-C3) | 1.358 | 1.383 | 1.379 | 1.377 | 1.358 | 1.383 | 1.378 | 1.377 | 1.382 | 1.382 | ||
| R(C3-C4) | 1.426 | 1.418 | 1.425 | 1.422 | 1.427 | 1.419 | 1.426 | 1.422 | 1.417 | 1.417 | ||
| R(N1-H6) | 0.993 | 1.012 | 1.009 | 1.008 | 0.992 | 1.011 | 1.008 | 1.007 | 0.996 | 1.000 | ||
| R(C2-H7) | 1.070 | 1.081 | 1.081 | 1.081 | 1.070 | 1.081 | 1.081 | 1.081 | 1.076 | 1.091 | ||
| R(C3-H8) | 1.071 | 1.082 | 1.082 | 1.082 | 1.071 | 1.082 | 1.082 | 1.082 | 1.077 | 1.088 | ||
| R(N11-N12) | 1.078 | 1.130 | 1.105 | 1.104 | 1.078 | 1.131 | 1.106 | 1.104 | … | … | ||
| R(N11..H6) | 2.659 | 2.379 | 2.421 | 2.518 | … | … | … | … | … | … | ||
| R(N11..H8) | … | … | … | … | 3.390 | 2.857 | 3.078 | 3.750 | … | … | ||
| R(N12..H9) | … | … | … | … | 3.398 | 2.847 | 3.124 | 3.801 | … | … | ||
| θ(N12-N11-H6) | 179.9 | 180.0 | 179.1 | 179.9 | … | … | … | … | … | … | ||
| θ(N11-H6-N1) | 179.9 | 180.0 | 179.0 | 179.9 | … | … | … | … | … | … | ||
| θ(N11-H8-C3) | … | … | … | … | 129.6 | 127.5 | 129.9 | 132.5 | … | … | ||
| θ(N12-H9-C4) | … | … | … | … | 128.7 | 126.2 | 126.3 | 129.0 | … | … | ||
| θ(N1-C2-C3) | 108.2 | 107.5 | 107.8 | 107.7 | 108.2 | 107.4 | 107.7 | 107.7 | 107.7 | 107.7 | ||
| θ(C2-C3-C4) | 107.1 | 107.5 | 107.4 | 107.3 | 107.1 | 107.5 | 107.4 | 107.4 | 107.4 | 107.4 | ||
| θ(C2-N1-C5) | 109.4 | 110.1 | 109.7 | 109.9 | 109.5 | 110.1 | 109.8 | 109.9 | 109.8 | 109.8 | ||
| θ(C2-N1-H6) | 125.3 | 125.0 | 125.2 | 125.1 | 125.3 | 124.9 | 125.1 | 125.0 | 125.1 | 125.1 | ||
| θ(N1-C2-H7) | 121.1 | 121.2 | 121.0 | 121.1 | 121.1 | 121.2 | 121.1 | 121.1 | 121.5 | 126.2 | ||
| θ(C2-C3-H8) | 126.0 | 125.6 | 125.8 | 125.8 | 126.0 | 125.5 | 125.8 | 125.7 | 125.5 | 126.3 | ||
| -E (310+) | 7.75308 | 8.73940 | 9.69222 | 9.56736 | 7.75208 | 8.73785 | 9.69047 | 9.5660 | ... | … | ||
| η | 6.1749 | 5.6730 | … | … | 6.4169 | 5.9499 | … | … | … | … | ||
| μ | -1.7204 | -2.0590 | … | … | -1.5540 | -1.8722 | … | … | … | … | ||
| μM | 2.1762 | 2.3045 | 2.3384 | 2.314 | 1.8956 | 1.8954 | 1.8839 | 1.9436 | … | … | ||
| RA | 9.2888 | 9.1632 | 9.1457 | 9.1864 | 8.0718 | 7.8749 | 7.9112 | 7.9445 | … | … | ||
| RB | 0.8167 | 0.8801 | 0.8722 | 0.8451 | 0.8816 | 1.0711 | 0.9727 | 0.7643 | … | … | ||
| RC | 0.7507 | 0.8030 | 0.7963 | 0.7739 | 0.7948 | 0.9429 | 0.8662 | 0.6972 | … | … | ||
In the second isomer, the nitrogen molecule bridges two hydrogen atoms of the pyrrole molecule. For this isomer also, there is no change in the geometrical parameters of the atoms in the ring structure. The nitrogen molecule interacts almost equally with the two hydrogen atoms H8 and H9 of the pyrrole molecule. The N11…H8 bond distance at HF/6-31G*, MP2/6-31G*, B3LYP/6-31G* and B3PW91/6-31G* is 3.390, 2.857, 3.078 and 3.750 Å, respectively. The bond distance of N12 and H9 atoms at the above levels of theory is 3.398, 2.844, 3.124 and 3.801Å. The optimized geometrical parameters, total energy, relative energy, interaction energy, rotational constants, chemical hardness and chemical potential for this complex are shown in Table 1. The first isomer which bound quasi linear to the NH group is found to be more stable. The previous calculation at MP2/6-31G* level of theory has indicated that the neutral N2..H2O complex [30] is more strongly bound than benzene…nitrogen complex [31]. In the pyrrole...nitrogen complex, the calculated NNH angle is comparable with the calculated value of phenol…nitrogen[2].

Table 2.
Relative energy orderings (in kcal/mol) (ERel) and interaction energy (in kcal/mol) after BSSE (EInt) for the various isomers of the pyrrole…nitrogen and the pyrrole..CO complexes
| Isomer | HF | MP2 | B3LYP | B3PW91 | ||||
| ERel | EInt | ERel | EInt | ERel | EIn | ERel | EInt | |
| Pyrrole…N2 | ||||||||
| Linear | 0.0 | -0.803 | 0.0 | -2.058 | 0.0 | -1.393 | 0.0 | -0.903 |
| T-Shaped | 0.628 | -0.176 | 0.973 | -1.086 | 1.098 | -0.282 | 0.853 | -0.044 |
| Pyrrole…CO | ||||||||
| Hydrogen bonded C end first | 0.0 | -1.512 | 0.0 | -3.150 | 0.0 | -2.711 | 0.0 | -2.146 |
| Above ring CO | +0.659 | -0.853 | +0.722 | -2.429 | +1.632 | -1.079 | +1.638 | -0.508 |
| Above ring OC | +0.559 | -0.960 | +0.753 | -2.391 | +1.650 | -1.048 | +1.682 | -0.452 |
| Hydrogen bonded O end first | +0.257 | -1.255 | +1.286 | -1.857 | +1.155 | -1.544 | +1.173 | -0.960 |
| T-shaped | +1.192 | -0.314 | +1.996 | -1.142 | +2.165 | -0.527 | +1.989 | -0.144 |
Interaction energies
The counterpoise corrected interaction energies at HF/6-31G*, MP2/6-31G*, B3LYP/6-31G* and B3PW91/6-31G* levels of theory are given in Table 2. The results show that the first isomer is more strongly bound than the second one. For both the isomers, the interaction energy calculated at MP2/6-31G* level of theory is much higher compared to all the other levels of theory. The reason is that the B3 functional at B3LYP and B3PW91 levels does not account for dispersion forces. The inclusion of dispersion forces in the MP2 level of theory gave a better estimate for the true interaction energy.
Chemical hardness and chemical potential
The development of well-defined exchange-correlational functionals has produced the results of molecular parameters, which are comparable in quality with experimental and ab initio results. According to the Maximum Hardness Principle (MHP), “there seems to be a rule of nature that molecules arrange themselves as hard as possible” [32]. Parr and Chattaraj [33] have proved this principle: The MHP is found to be valid even if the conditions of constant μ and v(r) are not satisfied. There is a great deal of qualitative evidence [32,34,35,36,37,38,39,40,41] and some semi quantitative evidence [42] supporting this principle. Nath et al [43] have studied the dissociation reaction H3N…HF→H3N + HF and the proton transfer reaction (F-H…Cl)-→(f…H-Cl)- and found that the reactions are found to obey the maximum hardness principle. Studies of chemical hardness and chemical potential on isomers and hardness profiles of hydrogen bonded systems [44] suggested that the extrema of the hardness profiles does not coincide well with the energy, but in some cases, the hardness profile follows the interaction path. In the present study, the chemical hardness η and chemical potential μ are calculated for these two isomers at HF/6-31G* and MP2/6-31G* levels of theory and are shown in Table 1. The higher chemical hardness value could not predict the energetically more stable isomer in both the levels of the theory. The same conclusion have been arrived for the number of cases of the hydrogen bonded systems.
PYRROLE-CARBON MONOXIDE COMPLEX
Geometries and energies
Due to the non-equivalence of the atoms of carbon monoxide, there are more number of possible structures for the pyrrole...CO complex compared to pyrrole...nitrogen. Moreover, carbon monoxide could bind more strongly than nitrogen due to its larger quadrupole moment [45] and additional small dipole moment (0.11 D). Two comparable isomers have also been observed for the H2O…CO complex in rare gas matrices, but only the “carbon in” isomer HOH…CO is observed in IR studies of neutral complexes in the gas phase [46]. The experimental and theoretical results of phenol…CO predict that the “carbon in” isomer is more stable where as “carbon out” phenol…OC structure occurs as a stable isomer with higher energy. In the above study, many possible complex structures have been considered in which CO molecule is: above the ring, perpendicular to the ring, inside the ring, T-shaped isomer and hydrogen bonded isomer. The HF, MP2, and B3LYP levels of theory with 6-31G* basis set have predicted that the hydrogen bonded ‘carbon in’ isomer is more stable and the hydrogen bonded ‘carbon out’ phenol…OC structure occurred as a stable isomer with higher energy. In the present study we have studied as many as pyrrole…CO isomers and the more reasonable isomers are shown in Fig. 2. All the structures are analogous to those of phenol…CO complex and can be grouped into two sets as CO and OC. Within each set there is a hydrogen-bonded isomer, a T-shaped isomer and an above ring isomer. The optimized parameters of these isomers at HF, MP2, B3LYP and B3PW91 levels of theory with 6-31G* basis set are given in Table 3. For the hydrogen-bonded isomers, the carbon monoxide is bound quasi linear with the =NH group of the pyrrole molecule. The carbon monoxide molecule formed as a T-shaped isomer is bound to the two hydrogen atoms of pyrrole in a trapezoidal arrangement. In the above ring isomers, the molecule CO lie approximately parallel above the center of pyrrole ring. The isomer bonding with carbon end first to the aromatic
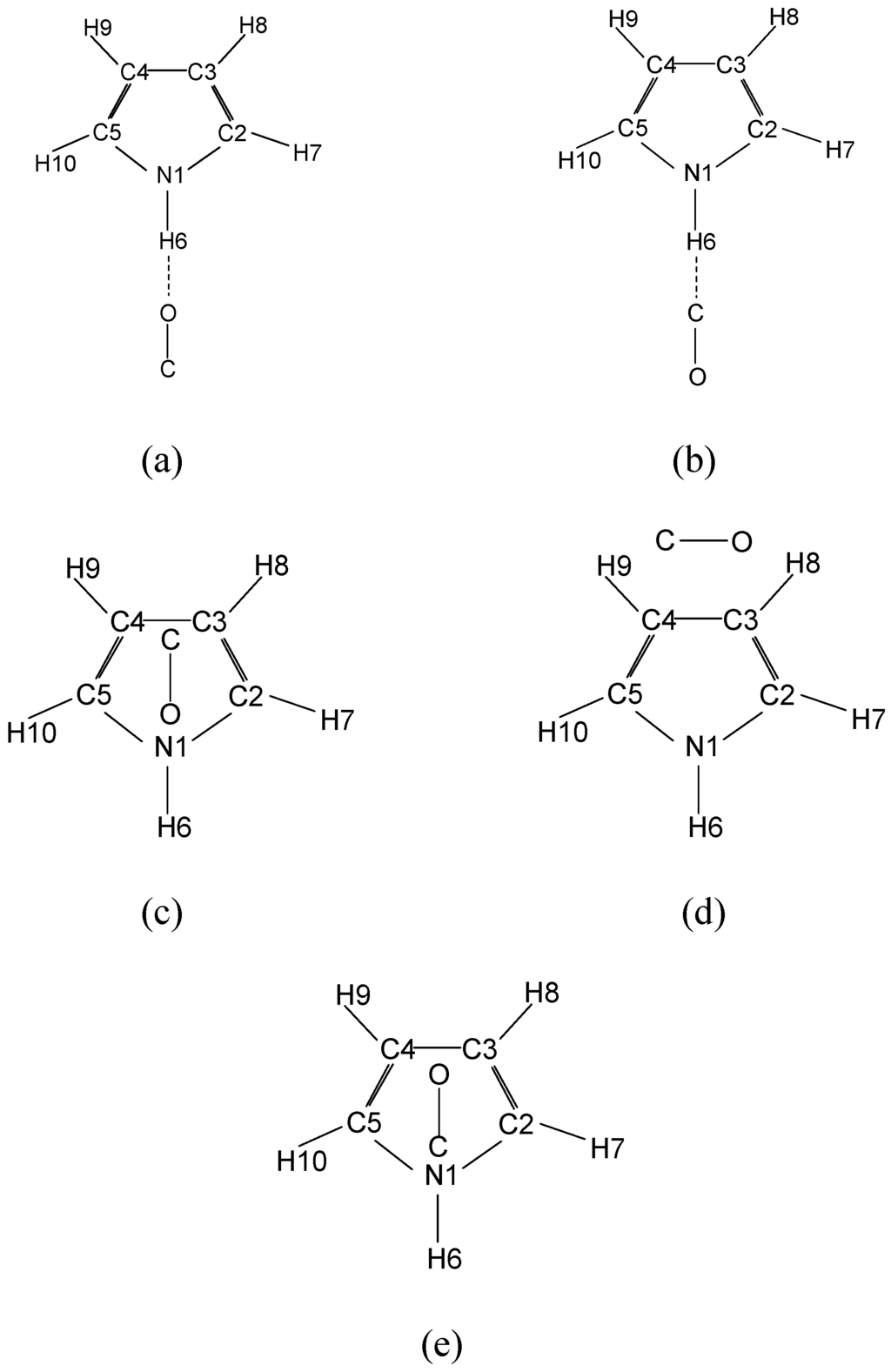

ring is of particular interest, since CO binding to aromatic ring via the Π-system is quite common in organometallic compounds. The most stable isomer for the pyrrole…CO complex at HF/6-31G*, MP2/6-31G*, B3LYP/6-31G* and B3PW91/6-31G* levels of theory is a hydrogen bonded structure with the carbon as the proton acceptor. In van der Waals complexes the hydrogen bond is along the axis of lone electron pair [47] and there are lone pairs on both carbon and oxygen atoms in CO, the most favored bonding orientation is through the carbon, due to its greater nucleophilicity. In many inorganic and organometallic compounds, this is the way the CO ligand binds. The other hydrogen bonded structure, with the oxygen as the proton acceptor is found to be the next most stable isomer and is found to be 0.257, 1.155 and 1.173 kcal/mol higher in energy at HF/6-31G*, B3LYP/6-31G* and B3PW91/6-31G* levels of theory, respectively. But MP2/6-31G* level of theory predicts the next most stable isomer as on the ring isomer. Table 2 gives the relative energy ordering of the isomers calculated at HF/6-31G*, MP2/6-31G*, B3LYP/6-31G* and B3PW91/6-31G* levels of theory and they differ in the subsequent ordering of the other isomers. Despite the differences, MP2/6-31G*, B3LYP/6-31G* and B3PW91/6-31G* levels of theory predict the two above ring isomers to be almost isoenergetic. The most obvious reason for the difference between MP2/6-31G* and the other two DFT methods with respect to the above ring isomer is that density functional theory does not properly treat the dispersion energy [48]. Dispersion energy will be the main interaction energy term for the above ring isomer, since there is very little dipole-dipole interaction. Accordingly, the MP2 values should be trusted more for the energy orderings than the DFT method values.

Figure 2.
Isomers of pyrrole...carbon monoxide complex (a) hydrogen bonded O end first, (b) hydrogen bonded C end first, (c) above ring OC, (d) T-shaped and (e) above ring CO.

Table 3.
Optimised geometrical parameters (bond length in Å, bond angle in degrees), total energy E (in Hartree) rotational constants RA,RB,,RC (in GHz), dipole moment μM (in Debye), chemical hardness η (in eV) and chemical potential μ (in eV) for Pyrrole..CO complex.
| Parameters | Hydrogen bonded O end first | Hydrogen bonded C end first | T-shaped | |||||||||||
| HF | MP2 | B3LYP | B3PW91 | HF | MP2 | B3LYP | B3PW91 | HF | MP2 | B3LYP | B3PW91 | |||
| R(N1-C2) | 1.362 | 1.373 | 1.375 | 1.370 | 1.362 | 1.372 | 1.375 | 1.370 | 1.363 | 1.373 | 1.375 | 1.371 | ||
| R(C2-C3) | 1.538 | 1.383 | 1.379 | 1.377 | 1.358 | 1.384 | 1.379 | 1.378 | 1.358 | 1.383 | 1.378 | 1.377 | ||
| R(C3-C4) | 1.426 | 1.418 | 1.425 | 1.422 | 1.426 | 1.418 | 1.425 | 1.422 | 1.427 | 1.419 | 1.426 | 1.422 | ||
| R(N1-H6) | 0.993 | 1.011 | 1.008 | 1.008 | 0.993 | 1.013 | 1.010 | 1.010 | 0.992 | 1.011 | 1.008 | 1.007 | ||
| R(C2-H7) | 1.070 | 1.081 | 1.081 | 1.081 | 1.070 | 1.081 | 1.081 | 1.081 | 1.070 | 1.081 | 1.080 | 1.081 | ||
| R(C3-H8) | 1.071 | 1.082 | 1.082 | 1.082 | 1.071 | 1.082 | 1.082 | 1.082 | 1.071 | 1.082 | 1.082 | 1.082 | ||
| R(C-O) | 1.115 | 1.152 | 1.139 | 1.138 | 1.112 | 1.149 | 1.135 | 1.135 | 1.114 | 1.151 | 1.138 | 1.137 | ||
| R(O…H6) | 2.443 | 2.295 | 2.290 | 2.404 | … | … | … | … | … | … | … | … | ||
| R(C…H6) | … | … | … | … | 2.618 | 2.400 | 2.388 | 2.416 | … | … | … | … | ||
| R(O…H8) | … | … | … | … | … | … | … | … | 3.222 | 2.864 | 3.029 | 3.129 | ||
| R(C…H9) | … | … | … | … | … | … | … | … | 3.397 | 2.925 | 3.068 | 3.485 | ||
| θ(C-O-H6) | 180.0 | 179.8 | 179.0 | 179.4 | … | … | … | … | … | … | … | … | ||
| θ(O-H6-N1) | 180.0 | 180.0 | 178.5 | 178.1 | … | … | … | … | … | … | … | … | ||
| θ(O-C-H6) | … | … | … | … | 179.7 | 180.0 | 179.5 | 179.6 | … | … | … | … | ||
| θ(C-H6-N1) | … | … | … | … | 179.7 | 180.0 | 178.8 | 178.5 | … | … | … | … | ||
| θ(O-H8-C3) | … | … | … | … | … | … | … | … | 137.5 | 131.3 | 134.5 | 137.5 | ||
| θ(C-H9-C4) | … | … | … | … | … | … | … | … | 120.6 | 123.3 | 121.4 | 121.4 | ||
| θ(N1-C2-C3) | 108.2 | 107.5 | 107.8 | 107.7 | 108.3 | 107.6 | 107.8 | 107.8 | 108.2 | 107.4 | 107.7 | 107.7 | ||
| θ(C2-C3-C4) | 107.1 | 107.5 | 107.4 | 107.3 | 107.1 | 107.4 | 107.3 | 107.3 | 107.1 | 107.5 | 107.4 | 107.4 | ||
| θ(C2-N1-C5) | 109.4 | 110.1 | 109.7 | 109.9 | 109.4 | 110.0 | 109.6 | 109.8 | 109.5 | 110.1 | 109.8 | 109.9 | ||
| θ(C2-N1-H6) | 125.3 | 125.0 | 125.1 | 125.0 | 125.3 | 125.0 | 125.1 | 125.0 | 125.3 | 124.9 | 125.1 | 125.0 | ||
| θ(N1-C2-H7) | 121.1 | 121.2 | 121.0 | 125.8 | 121.1 | 121.2 | 121.0 | 121.0 | 121.0 | 121.2 | 121.1 | 121.1 | ||
| θ(C2-C3-H8) | 126.0 | 125.6 | 125.8 | 3. | 126.0 | 125.6 | 125.6 | 125.8 | 125.9 | 125.5 | 125.7 | 125.7 | ||
| - E ( 320+) | 1.54772 | 2.50501 | 3.47779 | 3.35062 | 1.54813 | 2.50706 | 3.47963 | 3.35249 | 1.54623 | 2.50388 | 3.47618 | 3.34932 | ||
| η | 5.9779 | 5.7379 | … | … | 5.9495 | 5.6152 | 6.2379 | 5.9472 | … | … | ||||
| μ | -1.8708 | -2.0227 | … | … | -1.9306 | 2.1174 | … | … | -1.7439 | -1.8880 | … | … | ||
| μM | 2.5196 | 2.8116 | 2.3074 | 2.2559 | 2.0158 | 1.9413 | 2.5809 | 2.5850 | 1.9386 | 1.9752 | 1.8729 | 1.9354 | ||
| RA | 9.2905 | 9.1606 | 9.1456 | 9.1859 | 9.2917 | 9.1703 | 9.1540 | 9.1940 | 8.0561 | 7.8694 | 7.9209 | 7.9019 | ||
| RB | 0.8987 | 0.9301 | 0.9344 | 0.8990 | 0.8013 | 0.8435 | 0.8523 | 0.8458 | 0.9079 | 1.0494 | 0.9876 | 0.9038 | ||
| RC | 0.8194 | 0.8444 | 0.8478 | 0.8188 | 0.7377 | 0.7724 | 0.7797 | 0.7745 | 0.8159 | 0.9259 | 0.8781 | 0.8111 | ||
| Parameters | Above ring OC | Above ring CO | Experimental value | |||||||||||
| HF | MP2 | B3LYP | B3PW91 | HF | MP2 | B3LYP | B3PW91 | HF | MP2 | B3LYP | B3PW91 | |||
| R(N1-C2) | 1.362 | 1.737 | 1.375 | 1.370 | 1.362 | 1.373 | 1.374 | 1.370 | 1.370 | 1.370 | R(N1-C2) | 1.362 | ||
| R(C2-C3) | 1.358 | 1.383 | 1.378 | 1.377 | 1.358 | 1.383 | 1.379 | 1.378 | 1.382 | 1.382 | R(C2-C3) | 1.358 | ||
| R(C3-C4) | 1.427 | 1.419 | 1.426 | 1.423 | 1.427 | 1.418 | 1.425 | 1.422 | 1.417 | 1.417 | R(C3-C4) | 1.427 | ||
| R(N1-H6) | 0.993 | 1.011 | 1.008 | 1.007 | 0.993 | 1.011 | 1.008 | 1.007 | 0.996 | 1.000 | R(N1-H6) | 0.993 | ||
| R(C2-H7) | 1.070 | 1.081 | 1.080 | 1.081 | 1.070 | 1.081 | 1.080 | 1.081 | 1.076 | 1.091 | R(C2-H7) | 1.070 | ||
| R(C-O) | 1.115 | 1.152 | 1.139 | 1.138 | 1.114 | 1.151 | 1.139 | 1.138 | … | … | R(C-O) | 1.115 | ||
| R(O…N1) | 3.569 | 3.171 | 3.402 | 3.854 | … | … | … | … | … | … | R(O…N1) | 3.569 | ||
| R(C…N1) | … | … | … | … | 3.780 | 3.157 | 3.441 | 3.533 | … | … | R(C…N1) | … | ||
| R(O…C2) | 3.786 | 3.375 | 3.582 | 3.940 | … | … | … | … | … | … | R(O…C2) | 3.786 | ||
| R(C…C2) | … | … | … | … | 3.778 | 3.357 | 3.463 | 3.556 | … | … | R(C…C2) | … | ||
| R(O…C5) | 3.786 | 3.375 | 3.583 | 3.887 | … | … | … | … | … | … | R(O…C5) | 3.786 | ||
| R(C…C5) | … | … | … | … | 3.770 | 3.357 | 3.460 | 3.561 | … | … | R(C…C5) | … | ||
| θ(O-N1-C2) | 88.5 | 86.4 | 86.1 | 71.8 | … | … | … | … | … | … | θ(O-N1-C2) | 88.5 | ||
| θ(O-N1-C5) | 88.4 | 86.4 | 86.2 | 81.2 | … | … | … | … | … | … | θ(O-N1-C5) | 88.4 | ||
| θ(C-N1-C2) | … | … | … | … | 79.5 | 86.1 | 79.4 | 79.8 | … | … | θ(C-N1-C2) | … | ||
| θ(C-N1-C5) | … | … | … | … | 79.2 | 86.1 | 79.3 | 80.0 | … | ... | θ(C-N1-C5) | … | ||
| θ(N1-C2-C3) | 108.2 | 107.4 | 107.7 | 107.7 | 108.2 | 107.4 | 107.7 | 107.7 | 107.7 | 107.7 | θ(N1-C2-C3) | 108.2 | ||
| θ(C2-C3-C4) | 107.1 | 107.5 | 107.4 | 107.4 | 107.1 | 107.5 | 107.4 | 107.3 | 107.4 | 107.4 | θ(C2-C3-C4) | 107.1 | ||
| θ(C2-N1-C5) | 109.5 | 110.2 | 109.8 | 110.0 | 109.5 | 110.2 | 109.9 | 110.0 | 109.8 | 109.8 | θ(C2-N1-C5) | 109.5 | ||
| θ(C2-N1-H6) | 125.2 | 124.9 | 125.1 | 125.0 | 125.2 | 124.9 | 125.0 | 125.0 | 125.1 | 125.1 | θ(C2-N1-H6) | 125.2 | ||
| θ(N1-C2-H7) | 121.1 | 121.2 | 121.1 | 121.1 | 121.1 | 121.2 | 121.1 | 121.1 | 121.5 | 126.2 | θ(N1-C2-H7) | 121.1 | ||
| θ(C2-C3-H8) | 126.0 | 125.6 | 125.8 | 125.8 | 126.0 | 125.6 | 125.8 | 125.8 | 125.5 | 126.3 | θ(C2-C3-H8) | 126.0 | ||
| -E(320+) | 1.54724 | 2.50586 | 3.47700 | 3.34981 | 1.54708 | 2.50591 | 3.47703 | 3.34988 | … | … | -E(320+) | 1.54724 | ||
| η | 6.4693 | 6.2548 | … | … | 6.0285 | 6.2258 | … | … | … | … | η | 6.4693 | ||
| μ | -1.5655 | -1.6571 | … | … | -2.1776 | -1.7111 | … | … | … | … | μ | -1.5655 | ||
| μM | 1.6065 | 1.5201 | 1.9379 | 2.0099 | 2.1918 | 2.2042 | 1.9358 | 1.9385 | … | … | μM | 1.6065 | ||
| RA | 4.3337 | 4.2135 | 4.2129 | 4.2345 | 4.4545 | 4.2074 | 4.3764 | 4.3751 | … | … | RA | 4.3337 | ||
| RB | 1.5978 | 1.9415 | 1.7790 | 1.5561 | 1.4423 | 1.8846 | 1.6087 | 1.5519 | … | … | RB | 1.5978 | ||
| RC | 1.5599 | 1.8733 | 1.7227 | 1.5128 | 1.4239 | 1.8190 | 1.5840 | 1.5265 | … | … | RC | 1.5599 | ||
Interaction energies
The counterpoise corrected interaction energies for the pyrrole...CO isomers calculated at HF/6-31G*, MP2/6-31G*, B3LYP/6-31G* and B3PW91/6-31G* levels of theory are shown in Table 2. The isomer bonding carbon end first to the ring has more strongly bound than the other isomer in all the levels of theory. All the levels of theory except MP2/6-31G*, the isomer bonding oxygen end first to the ring has lesser interaction energy compared to that with CO bonding carbon end first to the ring where as MP2/6-31G* predicts the next lesser interaction energy for the ring isomer. All the levels of theory predict that the T-shaped isomer has the lowest interaction energy. The similar result was observed both experimentally and theoretically for phenol...CO isomer also [2].
Chemical hardness and chemical potential
The chemical hardness (η) and chemical potential (μ) are calculated at HF/6-31G* and MP2/6-31G* levels of theory for the pyrrole…CO complex and are shown in Table 2. This study gives the same conclusion as that of pyrrole...nitrogen. The higher chemical hardness value could not predict the energetically more stable isomer in both the levels of theory.
Conclusion
Two isomeric forms of pyrrole...nitrogen complex and the five isomeric forms of pyrrole...carbon monoxide complex have been studied using ab initio method at HF/6-31G*, MP2/6-31G* levels and at B3LYP/6-31G* and B3PW91/6-31G* levels of DFT method. The geometrical parameters, total energy, interaction energy, dipole moment, rotational constants, chemical hardness and chemical potential have been determined. Between the two isomers of pyrrole...nitrogen complex, the hydrogen bonded isomer is found to be more stable one. The same type of hydrogen bonded isomer with carbon end first is also found to be the most stable one among the five isomers of pyrrole...carbon monoxide. The higher chemical hardness value in both the complexes could not predict the energetically more stable isomer, concludes that the maximum hardness principle is not able to predict the most stable isomer for the hydrogen bonded complex.
Acknowledgments
The authors are thankful to Dr.K.S.Viswanathan, RCL, Indira Gandhi Center for Atomic Research (IGCAR), Kalpakkam, for allowing us to use the Gaussian 94W, Revision E.1 programme. One of the authors (R.K.) expresses his sincere thanks to the UGC, New Delhi, for the award of FIP fellowship during the IX plan period and he is thankful to the Management and the Principal, N. G. M. College, Pollachi, for allowing him to undergo this programme.
References
- Hobza, P.; Zahradnik, R. Chem. Rev. 1988, 88, 871.
- Chapman, D. M.; Müller-Dethlef, K. J. Chem. Phys. 1999, 111, 1955, and references there in.
- Webber, T.; Smith, A. M.; Riedle, E.; Neusser, H. J.; Schlag, E.W. Chem. Phys. Lett. 1990, 175, 79. [CrossRef]
- Brupbacher, T.; Bauder, A. J. Chem. Phys. 1993, 99, 9394.
- Hu, Y. H.; Lu, W. Y.; Yang, S. H. J. Chem. Phys. 1996, 105, 5305. [CrossRef]
- Brookes, M. D.; McKeller, A. R. W. J. Chem. Phys. 1998, 109, 5823. [CrossRef]
- Altman, R. S.; Marshal, M. D.; Klemperer, W. J. Chem. Phys. 1983, 79, 57. [CrossRef]
- Broquier, M.; Chevalier, M.; Picard-Bersellini, A. Mol. Phys. 1997, 91, 123.
- Goodwin, E. J.; Legon, A. C. J. Chem. Phys. 1985, 82, 4434.
- Lundell, J.; Räsänen, M. J. J. Chem. Phys. 1998, 109, 5823.
- Hains, S. R.; Dessent, C. E. H.; Müller-Dethlef, K. J. Chem. Phys. 1999, 111, 1947.
- Coblentz, W. W. Investigation of Infra-red Spectra; Carnegie Institute: Washington, DC, 1905; p. 331 p. [Google Scholar]
- Maroni-Ansidei, R.; Rolla, M. Atti. Accad. Lincei. 1938, 27, 410.
- Lord, R. C.; Miller, F. A. J. Chem. Phys. 1942, 10, 328.
- Mirone, P. Gazz. Chim. Ital. 1956, 86, 165.
- Sicorov, N. K.; Kalashnikova, L. P. Opt. Spectrosc. 1968, 26, 247.
- Lautié, A.; Novak, A. J. Chim. Phys. 1972, 69, 1332.
- Scott, D. W. J. Mol. Spectrosc. 1971, 37, 77. [CrossRef]
- Geidel, E.; Billes, F. J. Mol. Struct.(Theochem) 2000, 507, 75. [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Gill, P.M.W.; Johnson, B.G.; Robb, M.A.; Cheeseman, J.R.; Keith, T.; Petersson, G.A.; Montgomery, J.A.; Raghavachari, K.; Al-Laham, M.A.; Zakrzewski, V.G.; Ortiz, J.V.; Foresman, J.B.; Cioslowski, J.; Stefanov, B.B.; Nanayakkara, A.; Challacombe, M.; Peng, C.Y.; Ayala, P.Y.; Chen, W.; Wong, M.W.; Andres, J.L.; Replogle, E.S.; Gomperts, R.; Martin, R.L.; Fox, D.J.; Binkley, J.S.; Defrees, D.J.; Baker, J.; Stewart, J.P.; Head-Gordon, M.; Gonzalez, C.; Pople, J.A. Gaussian 94, Revision E.2; Gaussian: Pittsburgh PA, 1995. [Google Scholar]
- Becke, A. D. Phys. Rev. A 1988, 38, 3098. [CrossRef]
- Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B. 1988, 37, 785. [CrossRef]
- Perdew, J. P.; Wang, Y. Phys. Rev. B 1992, 45, 244. [CrossRef]
- Ghosh, S. K.; Berkowitz, M. J. Chem. Phys. 1985, 83, 2976.
- Berkowitz, M.; Ghosh, S. K.; Parr, R. G. J. Am. Chem. Soc. 1985, 107, 6811. [CrossRef]
- Paizs, B.; Suhai, S. J. Comp. Chem. 1998, 19, 575.
- Boys, S. F.; Bernardi, F. Mol. Phys. 1970, 19, 553.
- Nygaard, L.; Nielsen, J. T.; Kirchheiner, J.; Maltsen, G.; Rastrup-Andersen, J.; Sorensen, G. O. J. Mol. Struct. 1969, 3, 491. [CrossRef]
- Xie, Y.; Fan, K.; Boggs, J. E. Mol. Phys. 1986, 58, 401.
- Curtiss, L. A.; Eisgruber, C. L. J. Chem. Phys. 1984, 80, 2022.
- Wesolowski, T. A.; Parisel, O.; Ellinger, Y.; Weber, J. J. Phys. Chem. A. 1997, 101, 7818. [CrossRef]
- Pearson, R. G. J. Chem. Ed. 1987, 64, 561. [CrossRef]
- Parr, R. G.; Chattaraj, P. K. J. Am. Chem. Soc. 1991, 113, 1854. [CrossRef]
- Parr, R. G.; Pearson, R. G. J. Am. Chem. Soc. 1983, 105, 7512. [CrossRef]
- Bartell, L. S. J. Chem. Ed. 1968, 45, 754. [CrossRef]
- Burdett, J. K.; Coddens, B. K.; Kulkarni, G. V. Inorg. Chem. 1988, 27, 3259.
- Pearson, R. G. Chemtracts Inorg. Chem. 1991, 3, 317.
- Chattaraj, P. K. J. Indian Chem. Soc. 1992, 69, 173.
- Parr, R. G.; Chattaraj, P. K. J. Am. Chem. Soc. 1991, 96, 3283.
- Chattaraj, P. K.; Schilyer, P. V. R. J. Am. Chem. Soc. 1993, 116, 1069.
- Chattaraj, P. K.; Lee, H.; Parr, R. G. J. Am. Chem. Soc. 1991, 113, 1855. [CrossRef]
- Pearson, R. G. Proc. Natl. Acad. Sci. USA. 1988, 82, 6723.
- Nath, S.; Sannigrahi, A. B.; Chattaraj, P. K. J. Mol. Struct. (Theochem) 1994, 309, 65. [CrossRef]
- Arulmozhiraja, S.; Kolandaivel, P. Int. J. Quant. Chem. 1997, 64, 231.
- Graham, C.; Impie, D. A.; Raab, R. E. Mol. Phys. 1998, 93, 49.
- Lundell, J.; Latajka, Z. J. Phys. Chem. 1997, 101, 5004. [CrossRef]
- Legon, A. C.; Millen, D. J. Acc. Chem. Res. 1987, 20, 39. [CrossRef]
- Hobza, P.; Šponer, J.; Reschel, T. J. Comput. Chem. 1995, 16, 1315. [CrossRef]
© 2002 by MDPI (http://www.mdpi.org).