3-Hydroxypropionic Acid Enhances Hair Growth-Related Signaling in Human Follicle Dermal Papilla Cells via Activation of the Wnt/β-Catenin Pathway
Abstract
1. Introduction
2. Results
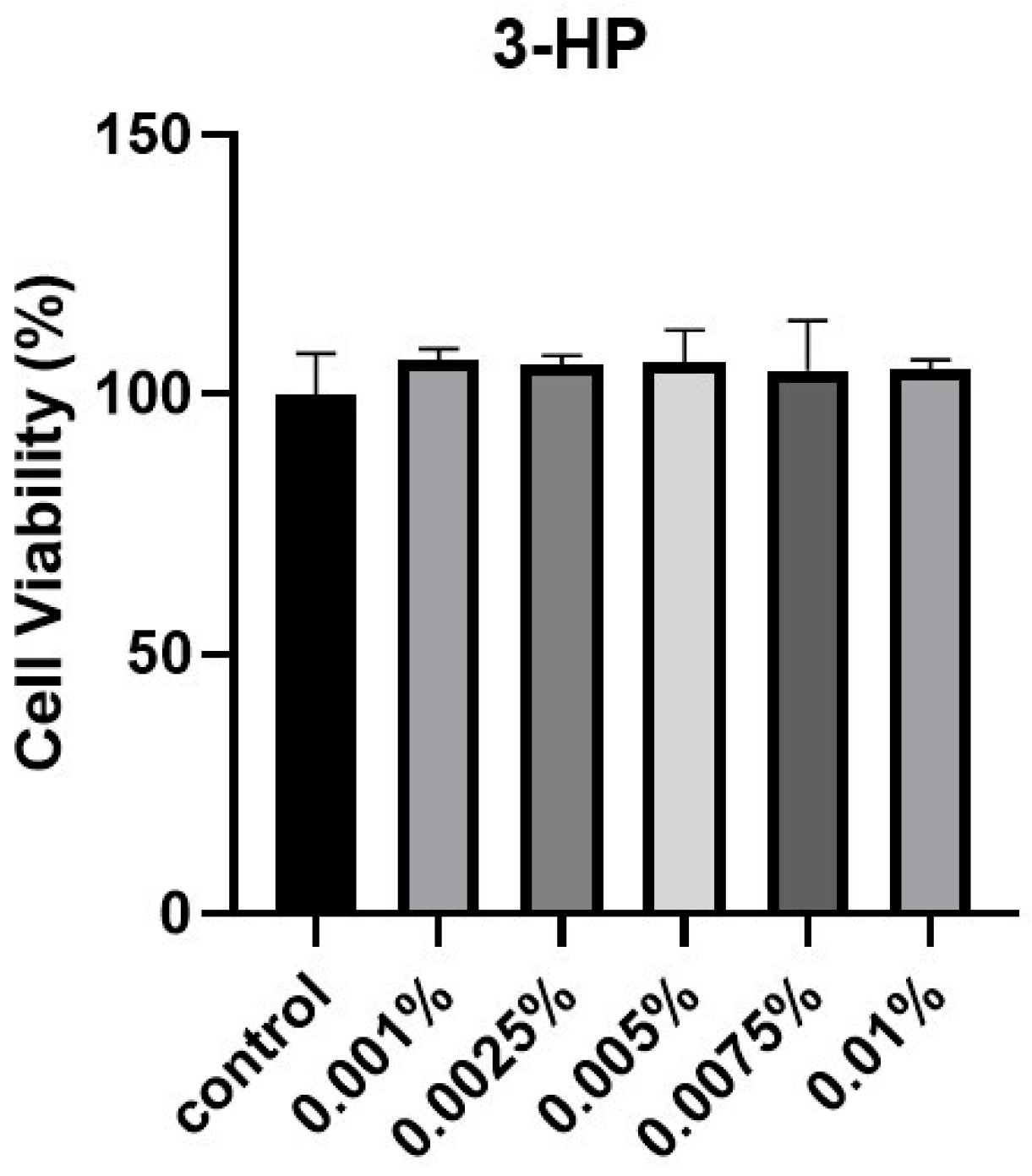
2.1. The Cell Viability of HDPCs Treated by 3-HP
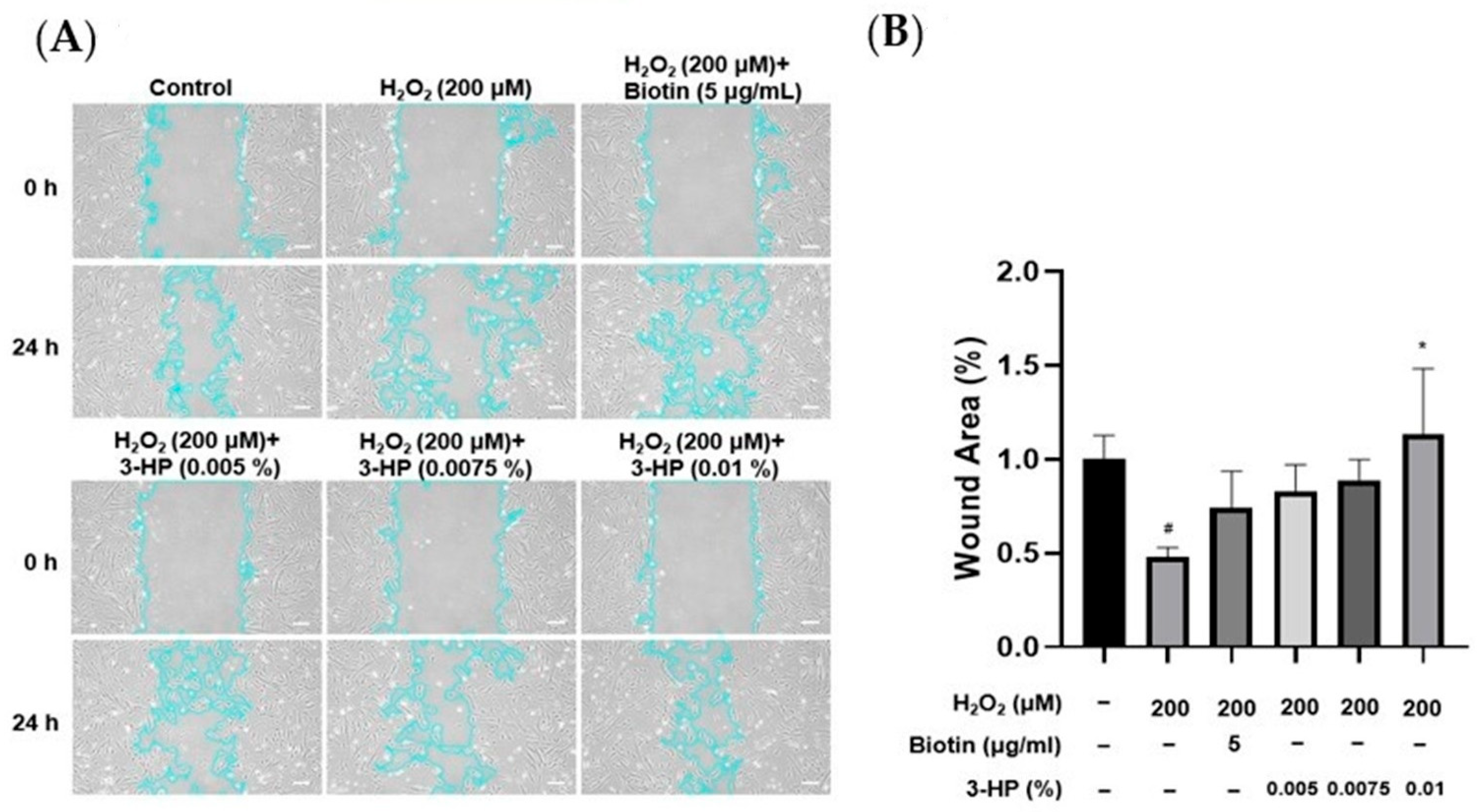
2.2. 3-HP Enhanced the Cell Wound Healing Ability of HFDPCs Damaged by H2O2
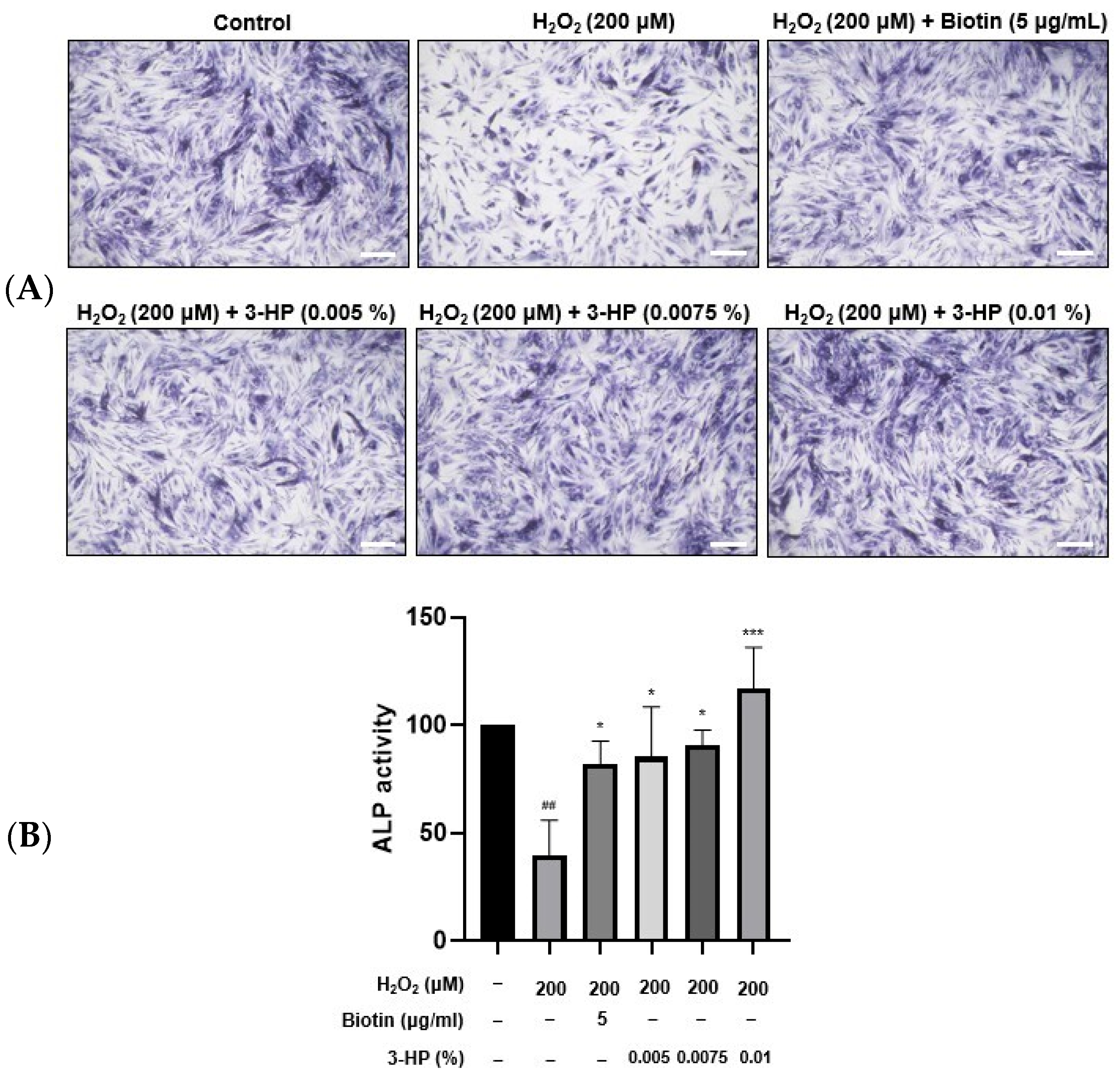
2.3. 3-HP Promoted ALP Expression in H2O2-Damaged HFDPCs
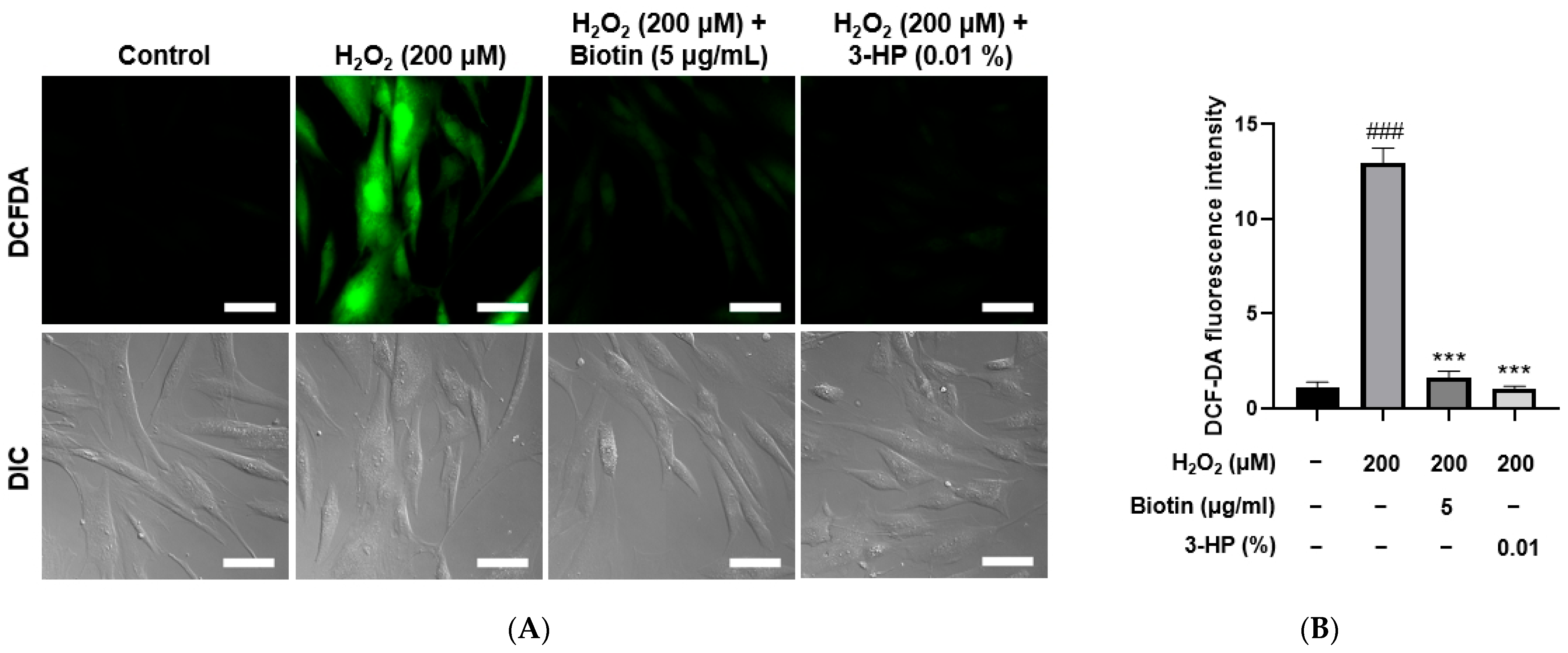
2.4. 3-HP Reduced ROS Levels in H2O2-Damaged HFDPCs
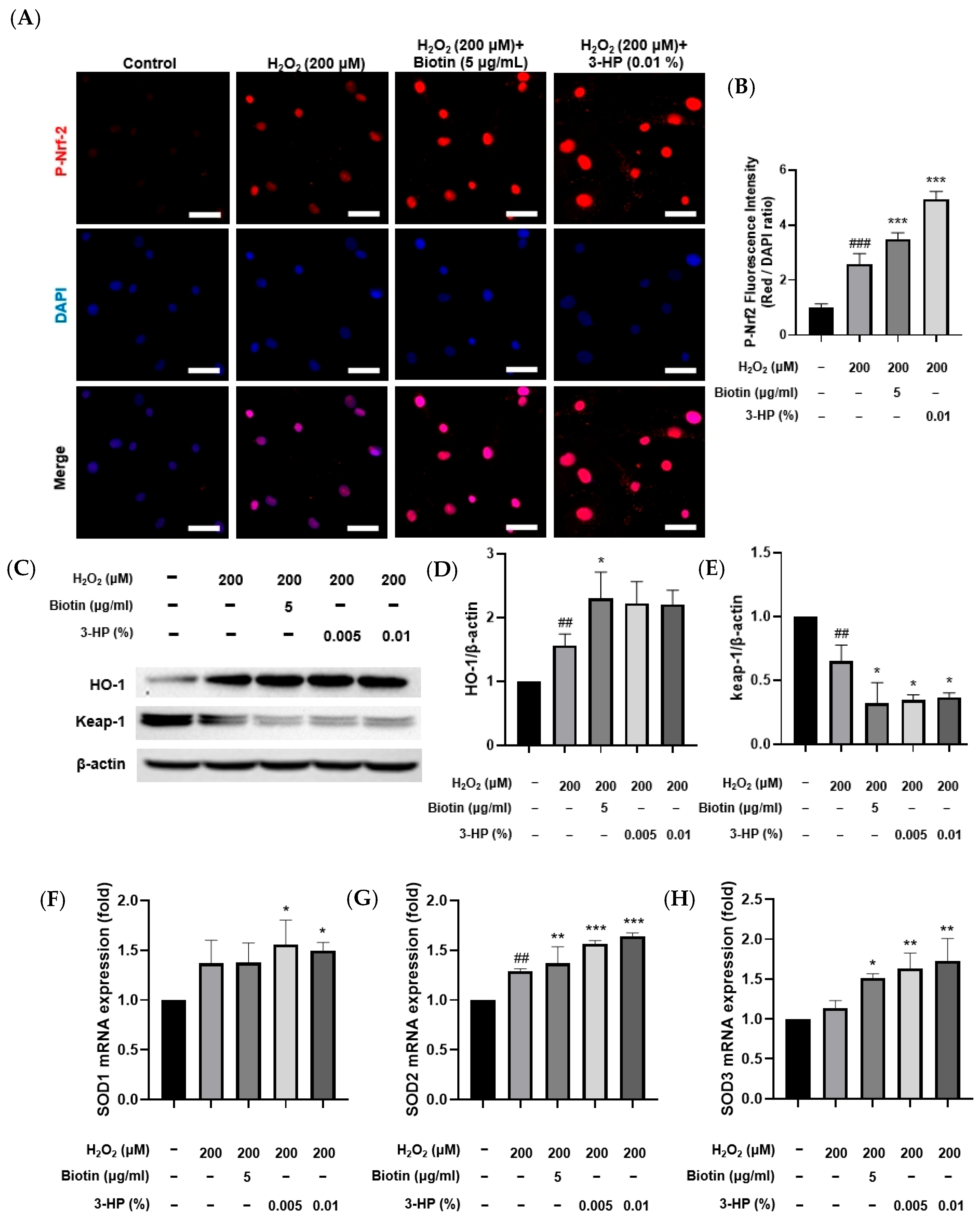
2.5. 3-HP Activated the Nrf2 Pathway in H2O2-Damaged HFDPCs
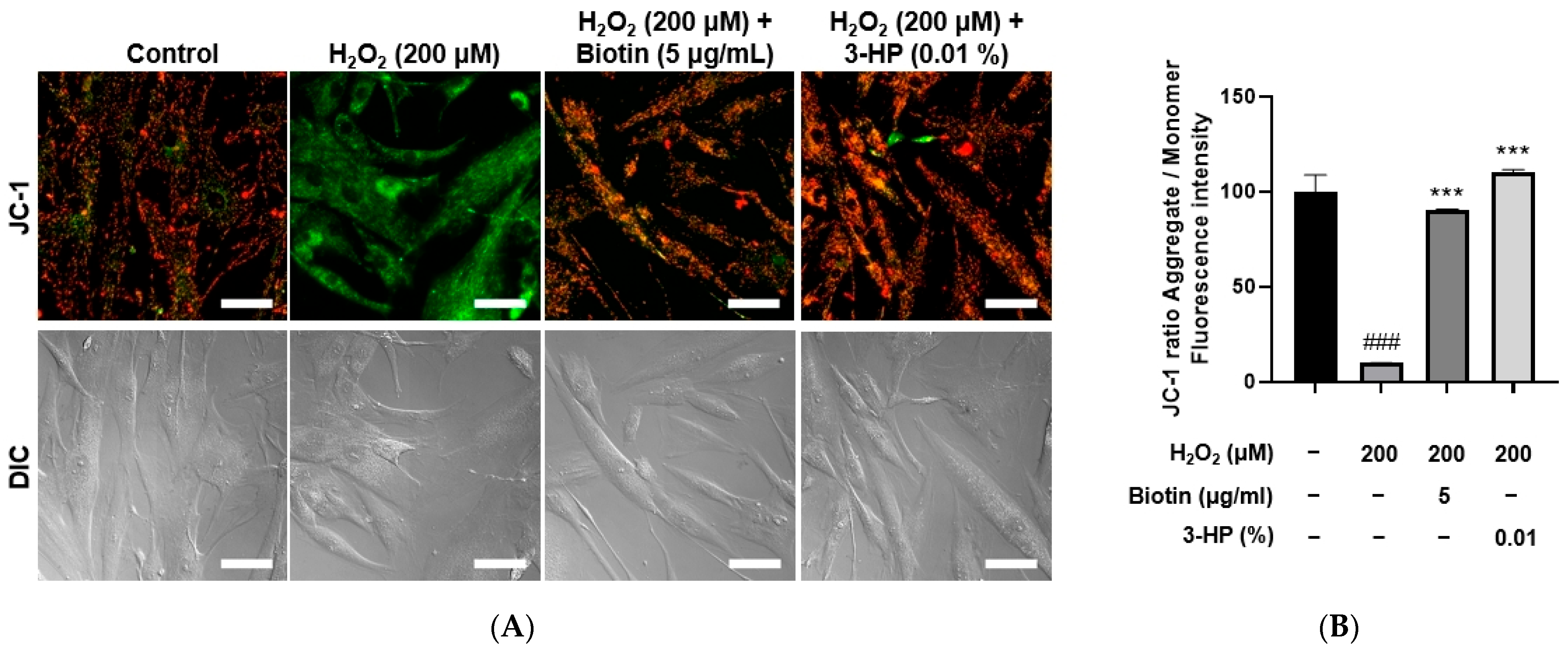
2.6. 3-HP Enhanced Mitochondrial Function in H2O2-Damaged HFDPCs
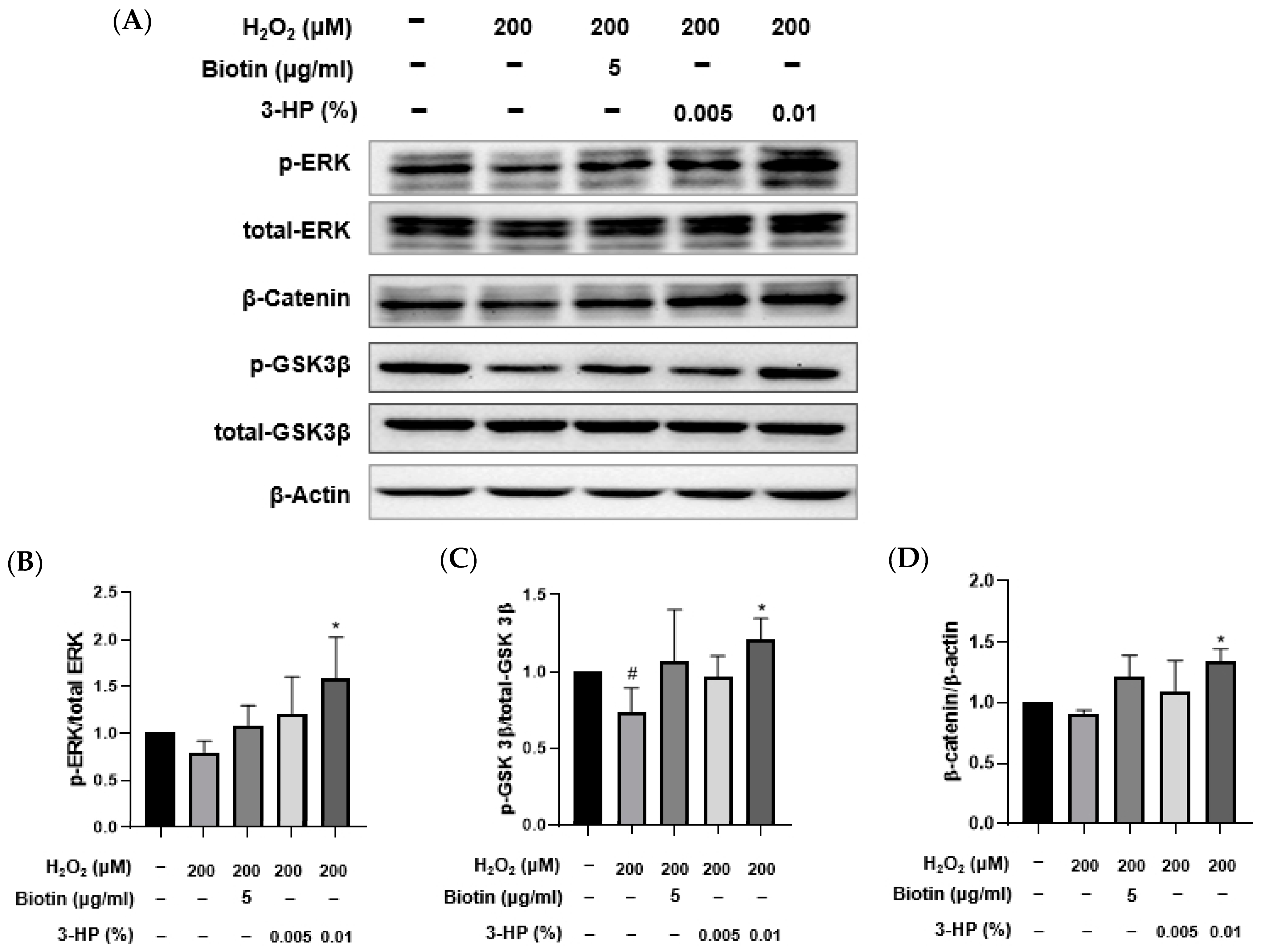
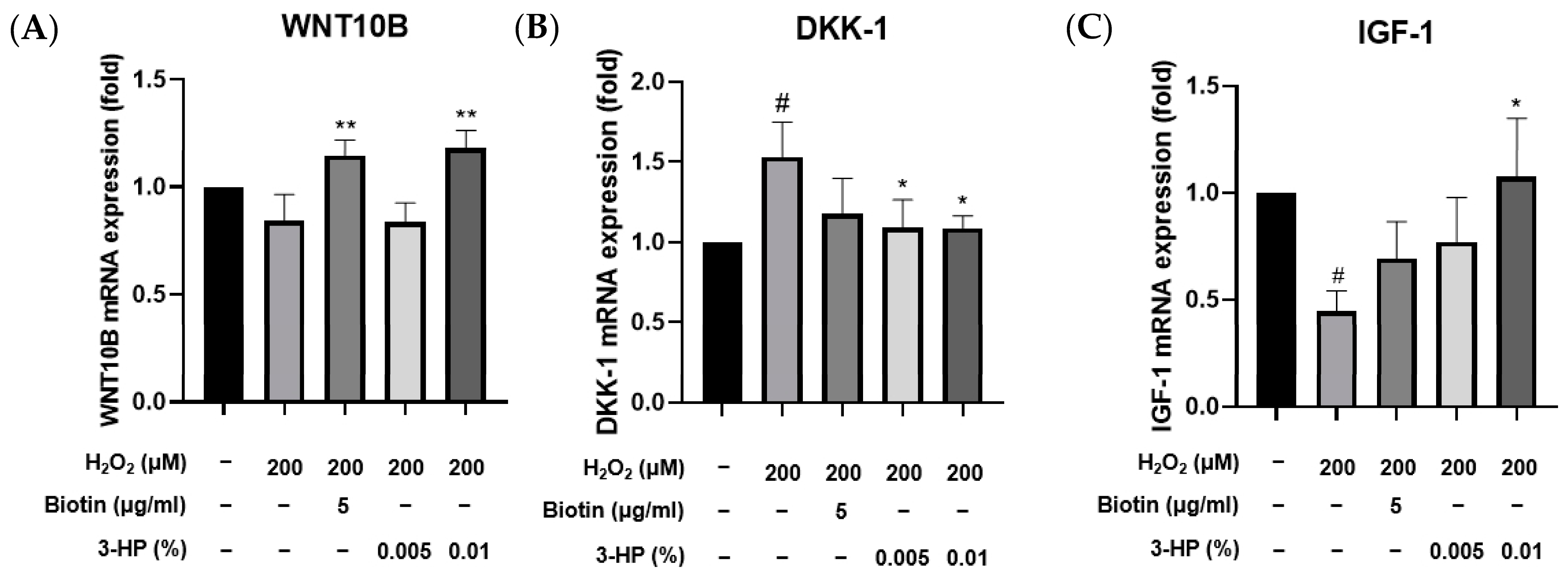
2.7. 3-HP Enhanced Hair Growth-Related Signaling Pathways in H2O2-Damaged HFDPCs
2.8. 3-HP Enhanced the Expression of Hair Growth-Related Genes in H2O2-Damaged HFDPCs
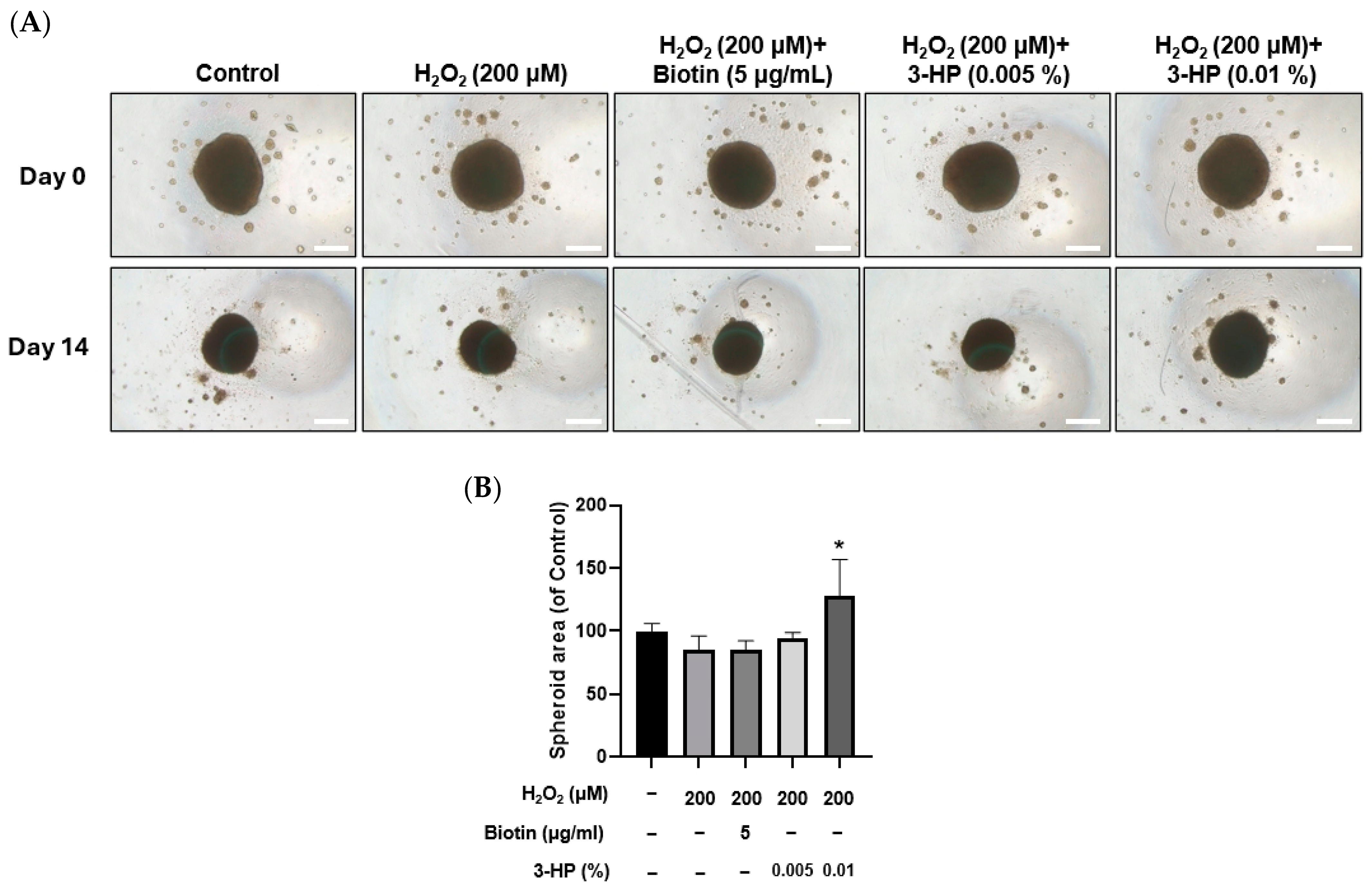
2.9. 3-HP Enhanced the Size of 3D Spheroids Formed by H2O2-Treated HFDPCs
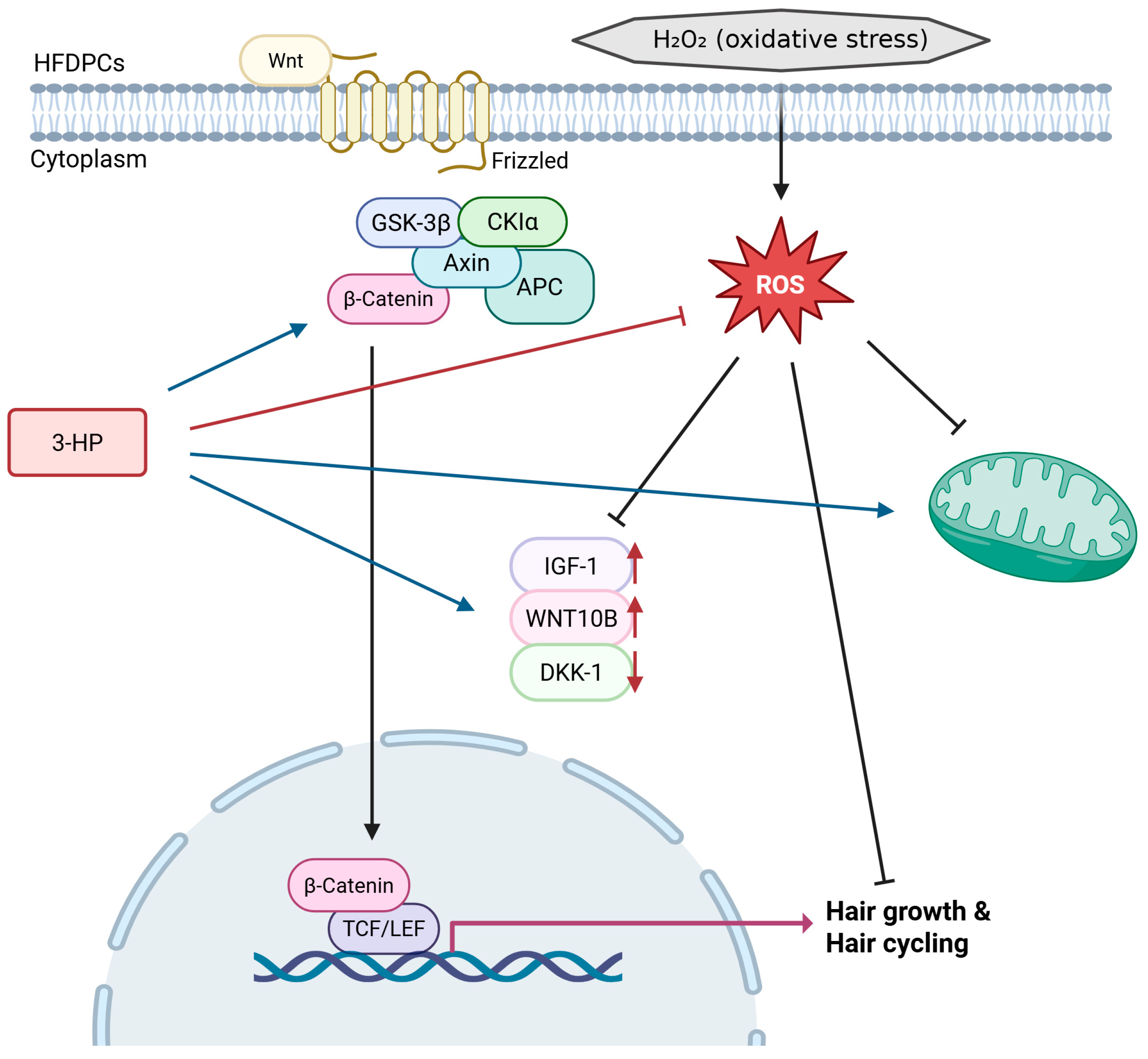
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Wound Healing Assay
4.5. Alkaline Phosphatase Staining (ALP) Assay
4.6. Measurement of Reactive Oxygen Species (ROS)
4.7. Immunofluorescence Analysis
4.8. Measurement of Mitochondrial Membrane Potential (JC-1)
4.9. Measurement of Mitochondrial ATP Content and Mitochondrial ROS
4.10. Western Blot Analysis
4.11. Real-Time Quantitative Reverse Transcriptase-Polymerase Chain Reaction
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HFDPCs | Human Follicle Dermal Papilla Cells |
| H2O2 | Hydrogen peroxide |
| DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| ROS | reactive oxygen species |
| FITC | Fluorescein isothiocyanate |
| RITC | Rhodamine B isothiocyanate |
| JC-1 | 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbo-cyanine iodide |
| IF | immunofluorescence |
| BCA | bicinchoninic acid |
| ERK | extracellular signal-regulated kinase |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| GSK3β | Glycogen synthase kinase-3 beta |
| IGF-1 | Insulin-like growth factor 1 |
| DKK-1 | Dickkopf-1 |
| 3-HP | 3-hydroxypropionic acid |
References
- Kim, S.M.; Kang, J.; Yoon, H.; Choi, Y.K.; Go, J.S.; Oh, S.K.; Ahn, M.; Kim, J.; Koh, Y.S.; Hyun, J.W.; et al. HNG, a Humanin Analogue, Promotes Hair Growth by Inhibiting Anagen-to-Catagen Transition. Int. J. Mol. Sci. 2020, 21, 4553. [Google Scholar] [CrossRef]
- Choi, B.Y. Hair-Growth Potential of Ginseng and Its Major Metabolites: A Review on Its Molecular Mechanisms. Int. J. Mol. Sci. 2018, 19, 2703. [Google Scholar] [CrossRef]
- Du, F.; Li, J.; Zhang, S.; Zeng, X.; Nie, J.; Li, Z. Oxidative stress in hair follicle development and hair growth: Signalling pathways, intervening mechanisms and potential of natural antioxidants. J. Cell Mol. Med. 2024, 28, e18486. [Google Scholar] [CrossRef]
- Chai, M.; Jiang, M.; Vergnes, L.; Fu, X.; de Barros, S.C.; Doan, N.B.; Huang, W.; Chu, J.; Jiao, J.; Herschman, H.; et al. Stimulation of Hair Growth by Small Molecules that Activate Autophagy. Cell Rep. 2019, 27, 3413–3421.e3. [Google Scholar] [CrossRef]
- Hou, C.; Miao, Y.; Wang, J.; Wang, X.; Chen, C.; Hu, Z. Collagenase IV plays an important role in regulating hair cycle by inducing VEGF, IGF-1, and TGF-β expression. Drug Des. Devel Ther. 2015, 9, 5373–5383. [Google Scholar] [CrossRef]
- Li, W.; Lu, Z.; Man, X.; Li, C.; Zhou, J.; Chen, J.; Yang, X.; Wu, X.; Cai, S.; Zheng, M. VEGF upregulates VEGF receptor-2 on human outer root sheath cells and stimulates proliferation through ERK pathway. Mol. Biol. Rep. 2012, 39, 8687–8694. [Google Scholar] [CrossRef]
- Lim, H.W.; Joo, H.; Jeon, C.Y.; Lee, Y.; Kim, M.; Shin, J.U.; Kim, J.; Kim, S.; Lee, S.; Lim, D.C.; et al. Anti-Hair Loss Effects of the DP2 Antagonist in Human Follicle Dermal Papilla Cells. Cosmetics 2024, 11, 177. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, M.; He, Y.; Liu, F.; Chen, L.; Xiong, X. Cellular Senescence: Ageing and Androgenetic Alopecia. Dermatology 2023, 239, 533–541. [Google Scholar] [CrossRef]
- Liang, A.; Fang, Y.; Ye, L.; Meng, J.; Wang, X.; Chen, J.; Xu, X. Signaling pathways in hair aging. Front. Cell Dev. Biol. 2023, 11, 1278278. [Google Scholar] [CrossRef]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative stress-associated senescence in dermal papilla cells of men with androgenetic alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef]
- Bertram, C.; Hass, R. Cellular responses to reactive oxygen species-induced DNA damage and aging. Biol. Chem. 2008, 389, 211–220. [Google Scholar] [CrossRef]
- Prie, B.E.; Iosif, L.; Tivig, I.; Stoian, I.; Giurcaneanu, C. Oxidative stress in androgenetic alopecia. J. Med. Life 2016, 9, 79–83. [Google Scholar]
- Chew, E.G.Y.; Lim, T.C.; Leong, M.F.; Liu, X.; Sia, Y.Y.; Leong, S.T.; Yan-Jiang, B.; Stoecklin, C.; Borhan, R.; Heilmann-Heimbach, S.; et al. Observations that suggest a contribution of altered dermal papilla mitochondrial function to androgenetic alopecia. Exp. Dermatol. 2022, 31, 906–917. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Haslam, I.S.; Jadkauskaite, L.; Szabo, I.L.; Staege, S.; Hesebeck-Brinckmann, J.; Jenkins, G.; Bhogal, R.K.; Lim, F.; Farjo, N.; Farjo, B.; et al. Oxidative Damage Control in a Human (Mini-) Organ: Nrf2 Activation Protects against Oxidative Stress-Induced Hair Growth Inhibition. J. Investig. Dermatol. 2017, 137, 295–304. [Google Scholar] [CrossRef]
- Jadkauskaite, L.; Coulombe, P.A.; Schafer, M.; Dinkova-Kostova, A.T.; Paus, R.; Haslam, I.S. Oxidative stress management in the hair follicle: Could targeting NRF2 counter age-related hair disorders and beyond? Bioessays 2017, 39, 1700029. [Google Scholar] [CrossRef]
- Park, S.; Lim, Y.J.; Kim, H.S.; Shin, H.; Kim, J.; Lee, J.N.; Lee, J.H.; Bae, S. Phloroglucinol Enhances Anagen Signaling and Alleviates H2O2-Induced Oxidative Stress in Human Dermal Papilla Cells. J. Microbiol. Biotechnol. 2024, 34, 812–827. [Google Scholar] [CrossRef]
- Huang, W.; Huang, Y.; Huang, K.; Chan, C.; Chiu, H.; Tsai, R.; Chan, J.; Lin, S. Stress-induced premature senescence of dermal papilla cells compromises hair follicle epithelial-mesenchymal interaction. J. Dermatol. Sci. 2017, 86, 114–122. [Google Scholar] [CrossRef]
- Han, L.; Liu, B.; Chen, X.; Chen, H.; Deng, W.; Yang, C.; Ji, B.; Wan, M. Activation of Wnt/β-catenin signaling is involved in hair growth-promoting effect of 655-nm red light and LED in in vitro culture model. Lasers Med. Sci. 2018, 33, 637–645. [Google Scholar] [CrossRef]
- Mehta, A.; Motavaf, M.; Raza, D.; McLure, A.J.; Osei-Opare, K.D.; Bordone, L.A.; Gru, A.A. Revolutionary Approaches to Hair Regrowth: Follicle Neogenesis, Wnt/ss-Catenin Signaling, and Emerging Therapies. Cells 2025, 14, 779. [Google Scholar] [CrossRef]
- L’Episcopo, F.; Tirolo, C.; Testa, N.; Caniglia, S.; Morale, M.C.; Impagnatiello, F.; Pluchino, S.; Marchetti, B. Aging-induced Nrf2-ARE pathway disruption in the subventricular zone drives neurogenic impairment in parkinsonian mice via PI3K-Wnt/β-catenin dysregulation. J. Neurosci. 2013, 33, 1462–1485. [Google Scholar] [CrossRef]
- Ebrahimi, K.B.; Cano, M.; Rhee, J.; Datta, S.; Wang, L.; Handa, J.T. Oxidative Stress Induces an Interactive Decline in Wnt and Nrf2 Signaling in Degenerating Retinal Pigment Epithelium. Antioxid. Redox Signal. 2018, 29, 389–407. [Google Scholar] [CrossRef]
- Tao, J.; Krutsenko, Y.; Moghe, A.; Singh, S.; Poddar, M.; Bell, A.; Oertel, M.; Singhi, A.D.; Geller, D.; Chen, X.; et al. Nuclear factor erythroid 2-related factor 2 and β-Catenin Coactivation in Hepatocellular Cancer: Biological and Therapeutic Implications. Hepatology 2021, 74, 741–759. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, B. Nrf2/Wnt resilience orchestrates rejuvenation of glia-neuron dialogue in Parkinson’s disease. Redox Biol. 2020, 36, 101664. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Sil, P.C. ROS-Influenced Regulatory Cross-Talk With Wnt Signaling Pathway During Perinatal Development. Front. Mol. Biosci. 2022, 9, 889719. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.; Cha, H.J.; Ahn, K.J.; An, I.; An, S.; Bae, S. Identification of microRNAs involved in growth arrest and cell death in hydrogen peroxide-treated human dermal papilla cells. Mol. Med. Rep. 2014, 10, 145–154. [Google Scholar] [CrossRef]
- Choi, Y.; Shin, J.Y.; Kim, J.; Kang, N.; Lee, S. Niacinamide Down-Regulates the Expression of DKK-1 and Protects Cells from Oxidative Stress in Cultured Human Dermal Papilla Cells. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1519–1528. [Google Scholar] [CrossRef]
- You, J.; Jang, Y.; Sim, J.; Ryu, D.; Cho, E.; Park, D.; Jung, E. Anti-Hair Loss Effect of Veratric Acid on Dermal Papilla Cells. Int. J. Mol. Sci. 2025, 26, 2240. [Google Scholar] [CrossRef]
- Lee, M.; Park, Y.B.; Moon, S.; Bok, S.H.; Kim, D.; Ha, T.; Jeong, T.; Jeong, K.; Choi, M. Hypocholesterolemic and antioxidant properties of 3-(4-hydroxyl)propanoic acid derivatives in high-cholesterol fed rats. Chem. Biol. Interact. 2007, 170, 9–19. [Google Scholar] [CrossRef]
- Liang, N.; Neuzil-Bunesova, V.; Tejnecky, V.; Ganzle, M.; Schwab, C. 3-Hydroxypropionic acid contributes to the antibacterial activity of glycerol metabolism by the food microbe Limosilactobacillus reuteri. Food Microbiol. 2021, 98, 103720. [Google Scholar] [CrossRef]
- Matsumoto, T.; Otani, T.; Yamada, R.; Ogino, H. Enhancing 3-hydroxypropionic acid production in Saccharomyces cerevisiae through enzyme localization within mitochondria. Biochem. Biophys. Res. Commun. 2023, 680, 1–6. [Google Scholar] [CrossRef]
- Kim, M.J.; Seong, K.; Kim, D.S.; Jeong, J.S.; Kim, S.Y.; Lee, S.; Yang, S.Y.; An, B. Minoxidil-loaded hyaluronic acid dissolving microneedles to alleviate hair loss in an alopecia animal model. Acta Biomater. 2022, 143, 189–202. [Google Scholar] [CrossRef]
- Park, S.; Kang, W.; Choi, D.; Son, B.; Park, T. Nonanal Stimulates Growth Factors via Cyclic Adenosine Monophosphate (cAMP) Signaling in Human Hair Follicle Dermal Papilla Cells. Int. J. Mol. Sci. 2020, 21, 8054. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, H.; Wu, X.; Song, Z.; Tang, H.; Gong, M.; Liu, L.; Li, F. Tetrathiomolybdate Decreases the Expression of Alkaline Phosphatase in Dermal Papilla Cells by Increasing Mitochondrial ROS Production. Int. J. Mol. Sci. 2023, 24, 3123. [Google Scholar] [CrossRef]
- Woo, W.; Zhen, H.H.; Oro, A.E. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes. Dev. 2012, 26, 1235–1246. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, J.; Shin, S.H.; Zahoor, M.; Kim, H.J.; Park, P.J.; Park, W.; Min, D.S.; Kim, H.; Choi, K. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS ONE 2012, 7, e34152. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Jang, Y.J.; Won, G.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Overexpression of alkaline phosphatase improves the hair-inductive capacity of cultured human dermal papilla spheres. J. Dermatol. Sci. 2019, 95, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Abreu, C.M.; Reis, R.L.; Marques, A.P. Dermal papilla cells and melanocytes response to physiological oxygen levels depends on their interactions. Cell Prolif. 2021, 54, e13013. [Google Scholar] [CrossRef]
- Kim, J.; Go, M.Y.; Jeon, C.Y.; Shin, J.U.; Kim, M.; Lim, H.W.; Shin, D.W. Pinitol Improves Diabetic Foot Ulcers in Streptozotocin-Induced Diabetes Rats Through Upregulation of Nrf2/HO-1 Signaling. Antioxidants 2024, 14, 15. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Xiong, X.; Zhu, H.; Chen, R.; Zhang, S.; Chen, G.; Jian, Z. Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Antioxidants 2022, 11, 2377. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Liu, S.; Pi, J.; Zhang, Q. Signal amplification in the KEAP1-NRF2-ARE antioxidant response pathway. Redox Biol. 2022, 54, 102389. [Google Scholar] [CrossRef]
- Avci, P.; Gupta, G.K.; Clark, J.; Wikonkal, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg. Med. 2014, 46, 144–151. [Google Scholar] [CrossRef]
- Blum, K.; Han, D.; Madigan, M.A.; Lohmann, R.; Braverman, E.R. “Cold” X5 Hairlaser used to treat male androgenic alopecia and hair growth: An uncontrolled pilot study. BMC Res. Notes 2014, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, J.W.; Kim, J.Y.; Shin, J.W.; Lee, S.; Huh, C. Low-level light therapy for androgenetic alopecia: A 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol. Surg. 2013, 39, 1177–1183. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio-Protocol 2019, 9, e3128. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yang, Y.; Xu, C.; Gao, S. A Flow Cytometry-based Assay for Measuring Mitochondrial Membrane Potential in Cardiac Myocytes After Hypoxia/Reoxygenation. J. Vis. Exp. 2018, 137, 57725. [Google Scholar] [CrossRef]
- Salin, K.; Villasevil, E.M.; Auer, S.K.; Anderson, G.J.; Selman, C.; Metcalfe, N.B.; Chinopoulos, C. Simultaneous measurement of mitochondrial respiration and ATP production in tissue homogenates and calculation of effective P/O ratios. Physiol. Rep. 2016, 4, e13007. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Baldassari, F.; De Marchi, E.; Karkucinska-Wieckowska, A.; Wieckowski, M.R.; Pinton, P. Methods to monitor and compare mitochondrial and glycolytic ATP production. Methods Enzymol. 2014, 542, 313–332. [Google Scholar] [CrossRef]
- Luz, A.L.; Lagido, C.; Hirschey, M.D.; Meyer, J.N. In Vivo Determination of Mitochondrial Function Using Luciferase-Expressing Caenorhabditis elegans: Contribution of Oxidative Phosphorylation, Glycolysis, and Fatty Acid Oxidation to Toxicant-Induced Dysfunction. Curr. Protoc. Toxicol. 2016, 69, 25.8.1–25.8.22. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kang, J.; Hyun, J.W.; Koh, Y.S.; Kang, J.; Hyun, C.; Yoon, K.; Lee, K.S.; Lee, C.M.; Kim, T.Y.; et al. Myristoleic Acid Promotes Anagen Signaling by Autophagy through Activating Wnt/β-Catenin and ERK Pathways in Dermal Papilla Cells. Biomol. Ther. 2021, 29, 211–219. [Google Scholar] [CrossRef]
- Huang, H.; Lin, H.; Huang, M. Lactoferrin promotes hair growth in mice and increases dermal papilla cell proliferation through Erk/Akt and Wnt signaling pathways. Arch. Dermatol. Res. 2019, 311, 411–420. [Google Scholar] [CrossRef]
- Kim, M.; Woo, J.; Kim, J.; Choi, M.; Shin, H.J.; Kim, Y.; Kim, J.; Shin, D.W. Iris germanica L. Rhizome-Derived Exosomes Ameliorated Dihydrotestosterone-Damaged Human Follicle Dermal Papilla Cells Through the Activation of Wnt/β-Catenin Pathway. Int. J. Mol. Sci. 2025, 26, 4070. [Google Scholar] [CrossRef]
- Tsai, F.; Li, C.; Wang, L.; Chen, M.; Lee, M.; Lin, Y.; Wang, C. Extracellular Signal-Regulated Kinase Mediates Ebastine-Induced Human Follicle Dermal Papilla Cell Proliferation. Biomed. Res. Int. 2019, 2019, 6360503. [Google Scholar] [CrossRef]
- Soma, T.; Fujiwara, S.; Shirakata, Y.; Hashimoto, K.; Kishimoto, J. Hair-inducing ability of human dermal papilla cells cultured under Wnt/β-catenin signalling activation. Exp. Dermatol. 2012, 21, 307–309. [Google Scholar] [CrossRef]
- Shin, D.W. The Molecular Mechanism of Natural Products Activating Wnt/β-Catenin Signaling Pathway for Improving Hair Loss. Life 2022, 12, 1856. [Google Scholar] [CrossRef]
- Nam, G.; Jo, K.; Park, Y.; Kawk, H.W.; Yoo, J.; Jang, J.D.; Kang, S.M.; Kim, S.; Kim, Y. Bacillus/Trapa japonica Fruit Extract Ferment Filtrate enhances human hair follicle dermal papilla cell proliferation via the Akt/ERK/GSK-3β signaling pathway. BMC Complement. Altern. Med. 2019, 19, 104. [Google Scholar] [CrossRef]
- Ki, G.; Kim, Y.; Lim, H.; Lee, E.; Choi, Y.; Seo, Y. Extremely Low-Frequency Electromagnetic Fields Increase the Expression of Anagen-Related Molecules in Human Dermal Papilla Cells via GSK-3β/ERK/Akt Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 784. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y.; Song, Z.; Hao, F.; Yang, X. Identification of Wnt/β-catenin signaling pathway in dermal papilla cells of human scalp hair follicles: TCF4 regulates the proliferation and secretory activity of dermal papilla cell. J. Dermatol. 2014, 41, 84–91. [Google Scholar] [CrossRef]
- Hwang, S.B.; Park, H.J.; Lee, B. Hair-Growth-Promoting Effects of the Fish Collagen Peptide in Human Dermal Papilla Cells and C57BL/6 Mice Modulating Wnt/β-Catenin and BMP Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 11904. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, Y.; Liu, H.; Liu, G.; Li, F. Wnt10b promotes hair follicles growth and dermal papilla cells proliferation via Wnt/β-Catenin signaling pathway in Rex rabbits. Biosci. Rep. 2020, 40, BSR20191248. [Google Scholar] [CrossRef]
- Zhu, N.; Yan, J.; Gu, W.; Yang, Q.; Lin, E.; Lu, S.; Cai, B.; Xia, B.; Liu, X.; Lin, C. Dermal papilla cell-secreted biglycan regulates hair follicle phase transit and regeneration by activating Wnt/β-catenin. Exp. Dermatol. 2024, 33, e14969. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, J.W.; Lee, H.; Lee, K.; Hwang, H.W.; Shin, H.; Byun, J.W.; Shin, J.; Choi, G.S. Dickkopf-related Protein 2 Promotes Hair Growth by Upregulating the Wnt/β-catenin Signaling Pathway in Human Dermal Papilla Cells. Ann. Dermatol. 2024, 36, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, K.; Ye, J.; Lian, X.; Yang, T. Wnt10b promotes growth of hair follicles via a canonical Wnt signalling pathway. Clin. Exp. Dermatol. 2011, 36, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Danek, P.; Kardosova, M.; Janeckova, L.; Karkoulia, E.; Vanickova, K.; Fabisik, M.; Lozano-Asencio, C.; Benoukraf, T.; Tirado-Magallanes, R.; Zhou, Q.; et al. β-Catenin-TCF/LEF signaling promotes steady-state and emergency granulopoiesis via G-CSF receptor upregulation. Blood 2020, 136, 2574–2587. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. L-ascorbic acid 2-phosphate represses the dihydrotestosterone-induced dickkopf-1 expression in human balding dermal papilla cells. Exp. Dermatol. 2010, 19, 1110–1112. [Google Scholar] [CrossRef]
- Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dickkopf 1 promotes regression of hair follicles. J. Investig. Dermatol. 2012, 132, 1554–1560. [Google Scholar] [CrossRef]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Investig. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef]
- Wang, Z.; Nan, W.; Si, H.; Wang, S.; Zhang, H.; Li, G. Pantothenic acid promotes dermal papilla cell proliferation in hair follicles of American minks via inhibitor of DNA Binding 3/Notch signaling pathway. Life Sci. 2020, 252, 117667. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Li, Z.; Gu, L.; Wang, Y.; Sung, C. Exogenous IGF-1 promotes hair growth by stimulating cell proliferation and down regulating TGF-β1 in C57BL/6 mice in vivo. Growth Horm. IGF Res. 2014, 24, 89–94. [Google Scholar] [CrossRef]
- Lei, M.; Guo, H.; Qiu, W.; Lai, X.; Yang, T.; Widelitz, R.B.; Chuong, C.; Lian, X.; Yang, L. Modulating hair follicle size with Wnt10b/DKK1 during hair regeneration. Exp. Dermatol. 2014, 23, 407–413. [Google Scholar] [CrossRef]
- Kovale, L.; Lee, S.; Song, M.; Lee, J.; Son, H.J.; Sung, Y.K.; Kwack, M.H.; Choe, W.; Kang, I.; Kim, S.S.; et al. Gynostemma pentaphyllum Hydrodistillate and Its Major Component Damulin B Promote Hair Growth-Inducing Properties In Vivo and In Vitro via the Wnt/β-Catenin Pathway in Dermal Papilla Cells. Nutrients 2024, 16, 985. [Google Scholar] [CrossRef]
- Higgins, C.A.; Chen, J.C.; Cerise, J.E.; Jahoda, C.A.B.; Christiano, A.M. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc. Natl. Acad. Sci. USA 2013, 110, 19679–19688. [Google Scholar] [CrossRef]
- Kazi, T.; Niibe, I.; Nishikawa, A.; Matsuzaki, T. Optimal stimulation toward the dermal papilla lineage can be promoted by combined use of osteogenic and adipogenic inducers. FEBS Open Bio 2020, 10, 197–210. [Google Scholar] [CrossRef]
- Bejaoui, M.; Oliva, A.K.; Ke, M.S.; Ferdousi, F.; Isoda, H. 3D Spheroid Human Dermal Papilla Cell as an Effective Model for the Screening of Hair Growth Promoting Compounds: Examples of Minoxidil and 3,4,5-Tri-O-caffeoylquinic acid (TCQA). Cells 2022, 11, 2093. [Google Scholar] [CrossRef]
- Andl, T.; Zhou, L.; Zhang, Y. The dermal papilla dilemma and potential breakthroughs in bioengineering hair follicles. Cell Tissue Res. 2023, 391, 221–233. [Google Scholar] [CrossRef]
- Lim, H.W.; Kim, H.J.; Jeon, C.Y.; Lee, Y.; Kim, M.; Kim, J.; Kim, S.R.; Lee, S.; Lim, D.C.; Park, H.D.; et al. Hair Growth Promoting Effects of 15-Hydroxyprostaglandin Dehydrogenase Inhibitor in Human Follicle Dermal Papilla Cells. Int. J. Mol. Sci. 2024, 25, 7485. [Google Scholar] [CrossRef]
- Jeon, C.Y.; Go, M.Y.; Kim, I.; Park, M.; Lee, H.W.; Kim, Y.; Shin, D.W. Hair Growth-Promoting Effects of Astragalus sinicus Extracts in Human Follicle Dermal Papilla Cells. Cosmetics 2025, 12, 6. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, B.; Deng, Z.; Wang, B.; Liu, F.; Li, J.; Shi, W.; Xie, H.; Hu, X.; Li, J. Mitochondrial aerobic respiration is activated during hair follicle stem cell differentiation, and its dysfunction retards hair regeneration. PeerJ 2016, 4, e1821. [Google Scholar] [CrossRef]
- Shin, J.Y.; Choi, Y.; Kim, J.; Park, S.Y.; Nam, Y.J.; Lee, S.Y.; Jeon, J.H.; Jin, M.H.; Lee, S. Polygonum multiflorum extract support hair growth by elongating anagen phase and abrogating the effect of androgen in cultured human dermal papilla cells. BMC Complement. Med. Ther. 2020, 20, 144–145. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef]
- Li, W.; Man, X.; Li, C.; Chen, J.; Zhou, J.; Cai, S.; Lu, Z.; Zheng, M. VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR-2-mediated activation of ERK. Exp. Cell Res. 2012, 318, 1633–1640. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, M.J.; Lee, W.; Pyo, J.; Shin, M.; Hwang, G.S.; Shin, D.; Kim, C.E.; Park, E.; Kang, K.S. Hair Growth Stimulation Effect of Centipeda minima Extract: Identification of Active Compounds and Anagen-Activating Signaling Pathways. Biomolecules 2021, 11, 976. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, J.; Jing, J.; Xue, D.; Liu, H.; Zheng, M.; Lu, Z. VEGF165 modulates proliferation, adhesion, migration and differentiation of cultured human outer root sheath cells from central hair follicle epithelium through VEGFR-2 activation in vitro. J. Dermatol. Sci. 2014, 73, 152–160. [Google Scholar] [CrossRef]
- Kim, J.; Shin, J.Y.; Choi, Y.; Kang, N.G.; Lee, S. Anti-Hair Loss Effect of Adenosine Is Exerted by cAMP Mediated Wnt/β-Catenin Pathway Stimulation via Modulation of Gsk3β Activity in Cultured Human Dermal Papilla Cells. Molecules 2022, 27, 2184. [Google Scholar] [CrossRef]
- Hwang, K.; Hwang, Y.; Lee, M.; Kim, N.; Roh, S.; Lee, Y.; Kim, C.D.; Lee, J.; Choi, K. Adenosine stimulates growth of dermal papilla and lengthens the anagen phase by increasing the cysteine level via fibroblast growth factors 2 and 7 in an organ culture of mouse vibrissae hair follicles. Int. J. Mol. Med. 2012, 29, 195–201. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, M.W.; Gil, H.; Chung, Y.J.; Kim, E.M. In vitro hair growth-promoting effect of Lgr5-binding octapeptide in human primary hair cells. J. Cosmet. Dermatol. 2024, 23, 986–998. [Google Scholar] [CrossRef]
- Betriu, N.; Jarrosson-Moral, C.; Semino, C.E. Culture and Differentiation of Human Hair Follicle Dermal Papilla Cells in a Soft 3D Self-Assembling Peptide Scaffold. Biomolecules 2020, 10, 684. [Google Scholar] [CrossRef]
- Hamida, O.B.; Kim, M.K.; Sung, Y.K.; Kim, M.K.; Kwack, M.H. Hair Regeneration Methods Using Cells Derived from Human Hair Follicles and Challenges to Overcome. Cells 2024, 14, 7. [Google Scholar] [CrossRef]
- Ji, S.; Zhu, Z.; Sun, X.; Fu, X. Functional hair follicle regeneration: An updated review. Signal Transduct. Target. Ther. 2021, 6, 66. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ntege, E.H.; Sunami, H.; Inoue, Y. Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature. Regen. Ther. 2022, 21, 527–539. [Google Scholar] [CrossRef]
- Kim, J.; Song, S.; Sung, J. Recent Advances in Drug Development for Hair Loss. Int. J. Mol. Sci. 2025, 26, 3461. [Google Scholar] [CrossRef]
- Duchi, S.; Rebollo Torregrosa, P.; Hajuj, A.; Molho, D.; Shkoor, R.; Saada, N.A.; Fernandez, D.G.; Goldstein, D.; Perez-Fernandez, A. The formulation and in vitro evaluation of WS Biotin, a novel encapsulated form of D-Biotin with improved water solubility for hair and skin treatment applications. Int. J. Cosmet. Sci. 2024, 46, 119–129. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of Hair Follicle Dermal Papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef]
- Patel, D.P.; Swink, S.M.; Castelo-Soccio, L. A Review of the Use of Biotin for Hair Loss. Skin. Appendage Disord. 2017, 3, 166–169. [Google Scholar] [CrossRef]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol. Ther. 2019, 9, 51–70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Jeon, C.Y.; Jo, Y.H.; Woo, S.A.; Lee, Y.; Jung, W.; Shin, D.W. 3-Hydroxypropionic Acid Enhances Hair Growth-Related Signaling in Human Follicle Dermal Papilla Cells via Activation of the Wnt/β-Catenin Pathway. Int. J. Mol. Sci. 2026, 27, 1480. https://doi.org/10.3390/ijms27031480
Jeon CY, Jo YH, Woo SA, Lee Y, Jung W, Shin DW. 3-Hydroxypropionic Acid Enhances Hair Growth-Related Signaling in Human Follicle Dermal Papilla Cells via Activation of the Wnt/β-Catenin Pathway. International Journal of Molecular Sciences. 2026; 27(3):1480. https://doi.org/10.3390/ijms27031480
Chicago/Turabian StyleJeon, Chae Young, Yun Hoo Jo, Seung A. Woo, Yura Lee, Woochul Jung, and Dong Wook Shin. 2026. "3-Hydroxypropionic Acid Enhances Hair Growth-Related Signaling in Human Follicle Dermal Papilla Cells via Activation of the Wnt/β-Catenin Pathway" International Journal of Molecular Sciences 27, no. 3: 1480. https://doi.org/10.3390/ijms27031480
APA StyleJeon, C. Y., Jo, Y. H., Woo, S. A., Lee, Y., Jung, W., & Shin, D. W. (2026). 3-Hydroxypropionic Acid Enhances Hair Growth-Related Signaling in Human Follicle Dermal Papilla Cells via Activation of the Wnt/β-Catenin Pathway. International Journal of Molecular Sciences, 27(3), 1480. https://doi.org/10.3390/ijms27031480






