The Role of Salicylic Acid in Activating Plant Stress Responses—Results of the Past Decade and Future Perspectives
Abstract
1. Introduction
2. Discussion
2.1. The Results of the Past Decade
2.1.1. Salicylic Acid and Signal Transduction
2.1.2. Salicylic Acid and Salt Stress
2.1.3. Salicylic Acid and Drought Stress
2.1.4. Salicylic Acid and Heavy Metal Stress
2.1.5. Salicylic Acid and Heat Stress
2.1.6. Salicylic Acid and Cold Stress
2.1.7. Salicylic Acid and Nutrient Stress
2.1.8. Salicylic Acid and Alkaline Stress
2.1.9. Salicylic Acid and Biotic Stress
2.1.10. Salicylic Acid and Oxidative Stress
2.1.11. Salicylic Acid and Oxidative Stress Caused by Xenobiotics
2.2. New Perspectives
2.2.1. Effects of Salicylic Acid on Secondary Metabolite Production
2.2.2. Salicylic Acid as Priming Effector
2.2.3. Salicylic Acid and Nanoparticles
2.2.4. Salicylic Acid in Combined Treatments
2.2.5. Salicylic Acid and Mycorrhiza Connections
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filgueiras, C.C.; Martins, A.D.; Pereira, R.V.; Willett, D.S. The Ecology of Salicylic Acid Signaling: Primary, Secondary, and Tertiary Effects with Applications in Agriculture. Int. J. Mol. Sci. 2019, 20, 5851. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Furukawa, J.; Sato, A.; Mizoguchi, T.; Miura, K. Abiotic Stress and Role of Salicylic Acid in Plants. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 235–251. ISBN 978-1-4614-0634-1. [Google Scholar]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic Acid-Induced Abiotic Stress Tolerance and Underlying Mechanisms in Plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic Acid to Decrease Plant Stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar] [CrossRef]
- Kaya, C.; Ugurlar, F.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Exploring the Synergistic Effects of Melatonin and Salicylic Acid in Enhancing Drought Stress Tolerance in Tomato Plants through Fine-Tuning Oxidative-Nitrosative Processes and Methylglyoxal Metabolism. Sci. Hortic. 2023, 321, 112368. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic Acid as a Safe Plant Protector and Growth Regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic Acid in Root Growth and Development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef]
- Klessig, D.F.; Tian, M.; Choi, H.W. Multiple Targets of Salicylic Acid and Its Derivatives in Plants and Animals. Front. Immunol. 2016, 7, 206. [Google Scholar] [CrossRef]
- Kavulych, Y.; Kobyletska, M.; Romanyuk, N.; Terek, O. Stress-Protective and Regulatory Properties of Salicylic Acid and Prospects of Its Use in Plant Production. Біoлoгічні студії/Stud. Biol. 2023, 17, 173–200. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, C.-B.; Ren, R.-M.; Jiang, J.-H. Salicylic Acid Had the Potential to Enhance Tolerance in Horticultural Crops against Abiotic Stress. Front. Plant Sci. 2023, 14, 1141918. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. The Importance of Salicylic Acid, Humic Acid, and Fulvic Acid on Crop Production. Lett. Drug Des. Discov. 2024, 21, 1465–1480. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Tak, Y.; Asthir, B. Salicylic Acid: A Key Signal Molecule Ameliorating Plant Stresses. Cereal Res. Commun. 2022, 50, 617–626. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic Acid: Biosynthesis and Signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef] [PubMed]
- Gondor, O.K.; Janda, T.; Soós, V.; Pál, M.; Majláth, I.; Adak, M.K.; Balázs, E.; Szalai, G. Salicylic Acid Induction of Flavonoid Biosynthesis Pathways in Wheat Varies by Treatment. Front. Plant Sci. 2016, 7, 1447. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, G.; Liu, C.; Li, H.; Chen, X.; Fu, Q.; Lin, Y.; Guo, B. Salicylic Acid, a Multifaceted Hormone, Combats Abiotic Stresses in Plants. Life 2022, 12, 886. [Google Scholar] [CrossRef]
- Liu, X.; Rockett, K.S.; Kørner, C.J.; Pajerowska-Mukhtar, K.M. Salicylic Acid Signalling: New Insights and Prospects at a Quarter-Century Milestone. Essays Biochem. 2015, 58, 101–113. [Google Scholar] [CrossRef]
- Zhao, P.; Lu, G.-H.; Yang, Y.-H. Salicylic Acid Signaling and Its Role in Responses to Stresses in Plants. In Mechanism of Plant Hormone Signaling Under Stress; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 413–441. ISBN 978-1-118-88902-2. [Google Scholar]
- Jia, X.; Wang, L.; Zhao, H.; Zhang, Y.; Chen, Z.; Xu, L.; Yi, K. The Origin and Evolution of Salicylic Acid Signaling and Biosynthesis in Plants. Mol. Plant 2023, 16, 245–259. [Google Scholar] [CrossRef]
- Chen, J.; Clinton, M.; Qi, G.; Wang, D.; Liu, F. Zheng Qing Fu, Reprogramming and Remodeling: Transcriptional and Epigenetic Regulation of Salicylic Acid-Mediated Plant Defense. J. Exp. Bot. 2020, 71, 5256–5268. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic Acid, a Multifaceted Hormone to Combat Disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Dempsey, D.M.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic Acid Biosynthesis and Metabolism. Arab. Book/Am. Soc. Plant Biol. 2011, 9, 0156. [Google Scholar] [CrossRef]
- Qi, G.; Chen, J.; Chang, M.; Chen, H.; Hall, K.; Korin, J.; Liu, F.; Wang, D.; Fu, Z.Q. Pandemonium Breaks Out: Disruption of Salicylic Acid-Mediated Defense by Plant Pathogens. Mol. Plant 2018, 11, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, S.; Ding, P.; Wang, D.; Cheng, Y.T.; He, J.; Zhang, Y. Control of Salicylic Acid Synthesis and Systemic Acquired Resistance by Two Members of a Plant-Specific Family of Transcription Factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18220–18225. [Google Scholar] [CrossRef] [PubMed]
- Verk, M.C.; Bol, J.F.; Linthorst, H.J. WRKY Transcription Factors Involved in Activation of SA Biosynthesis Genes. BMC Plant Biol 2011, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, J.; Zhu, Z.; Dong, X.; Wang, X.; Ren, G.; Kuai, B. TCP Transcription Factors Are Critical for the Coordinated Regulation of Isochorismate Synthase 1 Expression in Arabidopsis Thaliana. Plant J. 2015, 82, 151–162. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, Z.; Chu, Z. Emerging Roles of Salicylic Acid in Plant Saline Stress Tolerance. Int. J. Mol. Sci. 2023, 24, 3388. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Yan, S.; Saleh, A.; Wang, W.; Ruble, J.; Oka, N.; Dong, X. NPR3 and NPR4 Are Receptors for the Immune Signal Salicylic Acid in Plants. Nature 2012, 486, 228–232. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, D.; Chu, J.Y.; Boyle, P.; Wang, Y.; Brindle, I.D.; Després, C. The Arabidopsis NPR1 Protein Is a Receptor for the Plant Defense Hormone Salicylic Acid. Cell Rep. 2012, 1, 639–647. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, T.; Ao, K.; Peng, Y.; Zhang, Y.; Li, X.; Zhang, Y. Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell 2018, 173, 1454–1467. [Google Scholar] [CrossRef]
- Janda, M.; Ruelland, E. Magical Mystery Tour: Salicylic Acid Signalling. Environ. Exp. Bot. 2015, 114, 117–128. [Google Scholar] [CrossRef]
- Hernández, J.A.; Diaz-Vivancos, P.; Barba-Espín, G.; Clemente-Moreno, M.J. On the Role of Salicylic Acid in Plant Responses to Environmental Stresses. In Salicylic Acid: A Multifaceted Hormone; Nazar, R., Iqbal, N., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 17–34. ISBN 978-981-10-6068-7. [Google Scholar]
- Lai, Y.-S.; Renna, L.; Yarema, J.; Ruberti, C.; He, S.Y.; Brandizzi, F. Salicylic Acid-Independent Role of NPR1 Is Required for Protection from Proteotoxic Stress in the Plant Endoplasmic Reticulum. Proc. Natl. Acad. Sci. USA 2018, 115, E5203–E5212. [Google Scholar] [CrossRef]
- Poór, P. Effects of Salicylic Acid on the Metabolism of Mitochondrial Reactive Oxygen Species in Plants. Biomolecules 2020, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Ruberti, C.; Gong, Z.; Brandizzi, F. CPR5 Modulates Salicylic Acid and the Unfolded Protein Response to Manage Tradeoffs between Plant Growth and Stress Responses. Plant J. 2017, 89, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Pokotylo, I.; Kravets, V.; Ruelland, E. Salicylic Acid Binding Proteins (SABPs): The Hidden Forefront of Salicylic Acid Signalling. Int. J. Mol. Sci. 2019, 20, 4377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Y.T.; Qu, N.; Zhao, Q.; Bi, D.; Li, X. Negative Regulation of Defense Responses in Arabidopsis by Two NPR1 Paralogs. Plant J. 2006, 48, 647–656. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Klessig, D.F. How Does the Multifaceted Plant Hormone Salicylic Acid Combat Disease in Plants and Are Similar Mechanisms Utilized in Humans? BMC Biol. 2017, 15, 23. [Google Scholar] [CrossRef]
- Rekhter, D.; Lüdke, D.; Ding, Y.; Feussner, K.; Zienkiewicz, K.; Lipka, V.; Wiermer, M.; Zhang, Y.; Feussner, I. Isochorismate-Derived Biosynthesis of the Plant Stress Hormone Salicylic Acid. Science 2019, 365, 498–502. [Google Scholar] [CrossRef]
- Torrens-Spence, M.P.; Bobokalonova, A.; Carballo, V.; Glinkerman, C.M.; Pluskal, T.; Shen, A.; Weng, J.K. PBS3 and EPS1 Complete Salicylic Acid Biosynthesis from Isochorismate in Arabidopsis. Mol. Plant 2019, 12, 1577–1586. [Google Scholar] [CrossRef]
- Song, W.; Shao, H.; Zheng, A.; Zhao, L.; Xu, Y. Advances in Roles of Salicylic Acid in Plant Tolerance Responses to Biotic and Abiotic Stresses. Plants 2023, 12, 3475. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, X.; Wei, J.; Li, W.; Wang, H.; Xu, Y.; Yang, Z.; Xu, C.; Li, P. Primary Root Response to Combined Drought and Heat Stress Is Regulated via Salicylic Acid Metabolism in Maize. BMC Plant Biol. 2022, 22, 417. [Google Scholar] [CrossRef]
- Ahmad, F.; Singh, A.; Kamal, A. Chapter 23—Salicylic Acid–Mediated Defense Mechanisms to Abiotic Stress Tolerance. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 355–369. ISBN 978-0-12-816451-8. [Google Scholar]
- Belt, K.; Huang, S.; Thatcher, L.F.; Casarotto, H.; Singh, K.B.; Van Aken, O.; Millar, A.H. Salicylic Acid-Dependent Plant Stress Signaling via Mitochondrial Succinate Dehydrogenase. Plant Physiol. 2017, 173, 2029–2040. [Google Scholar] [CrossRef]
- Santisree, P.; Jalli, L.C.L.; Bhatnagar-Mathur, P.; Sharma, K.K. Emerging Roles of Salicylic Acid and Jasmonates in Plant Abiotic Stress Responses. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 342–373. ISBN 978-1-119-55215-4. [Google Scholar]
- Wang, Y.; Hu, Q.; Wu, Z.; Wang, H.; Han, S.; Jin, Y.; Yang, W. HISTONE DEACETYLASE 6 Represses Pathogen Defence Responses in Arabidopsis Thaliana. Plant Cell Environ. 2017, 40, 2972–2986. [Google Scholar] [CrossRef] [PubMed]
- Buszewicz, D.; Archacki, R.; Palusiński, A.; Kotliński, M.; Fogtman, A.; Iwanicka-Nowicka, R.; Koblowska, M.K. HD2C Histone Deacetylase and a SWI/SNF Chromatin Remodelling Complex Interact and Both Are Involved in Mediating the Heat Stress Response in Arabidopsis. Plant Cell Environ. 2016, 39, 2108–2122. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ding, Y.; Sun, X.; Xie, S.; Wang, D.; Liu, X.; Zhou, D.X. Histone Deacetylase HDA9 Negatively Regulates Salt and Drought Stress Responsiveness in Arabidopsis. J. Exp. Bot. 2016, 67, 1703–1713. [Google Scholar] [CrossRef]
- Jin, H.; Choi, S.M.; Kang, M.J.; Yun, S.H.; Kwon, D.J.; Noh, Y.S.; Noh, B. Salicylic Acid-Induced Transcriptional Reprogramming by the HAC–NPR1–TGA Histone Acetyltransferase Complex in Arabidopsis. Nucleic Acids Res. 2018, 46, 11712–11725. [Google Scholar] [CrossRef]
- Dutta, A.; Choudhary, P.; Caruana, J.; Raina, R. JMJ 27, an Arabidopsis H3K9 Histone Demethylase, Modulates Defense against Pseudomonas Syringae and Flowering Time. Plant J. 2017, 91, 1015–1028. [Google Scholar] [CrossRef]
- López Sánchez, A.; Stassen, J.H.; Furci, L.; Smith, L.M.; Ton, J. The Role of DNA (de) Methylation in Immune Responsiveness of Arabidopsis. Plant J. 2016, 88, 361–374. [Google Scholar] [CrossRef]
- Weiberg, A.; Wang, M.; Bellinger, M.; Jin, H. Small RNAs: A New Paradigm in Plant-Microbe Interactions. Annu. Rev. Phytopathol. 2014, 52, 495–516. [Google Scholar] [CrossRef]
- Spoel, S.H.; Dong, X. Salicylic Acid in Plant Immunity and Beyond. Plant Cell 2024, 36, 1451–1464. [Google Scholar] [CrossRef]
- Hong, J.; Meng, K.; Thomas, H.R.; Yang, Y.; Williams, B.; Kang, H.; Zhou, Y.; Hong, J.; Meng, K.; Thomas, H.R.; et al. Reframing Agriculture by Light: The Role of Light-Mediated Jasmonates/Salicylic Acid Regulation in Plant Defense, Development and Beyond. Veg. Res. 2024, 4, e027. [Google Scholar] [CrossRef]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic Acid in Plant Salinity Stress Signalling and Tolerance. Plant Growth Regul. 2015, 76, 25–40. [Google Scholar] [CrossRef]
- Bhuvaneshwari, V. Salicylic Acid Induced Salt Stress Tolerance in Plants. Int. J. Plant Biol. Res. 2017, 5, 1067–1073. [Google Scholar]
- Hoque, T.S.; Sohag, A.A.M.; Burritt, D.J.; Hossain, M.A. Salicylic Acid-Mediated Salt Stress Tolerance in Plants. In Plant Phenolics in Sustainable Agriculture: Volume 1; Lone, R., Shuab, R., Kamili, A.N., Eds.; Springer: Singapore, 2020; pp. 1–38. ISBN 9789811548901. [Google Scholar]
- Sharma, A.; Kohli, S.K.; Khanna, K.; Ramakrishnan, M.; Kumar, V.; Bhardwaj, R.; Brestic, M.; Skalicky, M.; Landi, M.; Zheng, B. Salicylic Acid: A Phenolic Molecule with Multiple Roles in Salt-Stressed Plants. J. Plant Growth Regul. 2023, 42, 4581–4605. [Google Scholar] [CrossRef]
- Mimouni, H.; Wasti, S.; Manaa, A.; Gharbi, E.; Chalh, A.; Vandoorne, B.; Lutts, S.; Ahmed, H.B. Does Salicylic Acid (SA) Improve Tolerance to Salt Stress in Plants? A Study of SA Effects On Tomato Plant Growth, Water Dynamics, Photosynthesis, and Biochemical Parameters. OMICS A J. Integr. Biol. 2016, 20, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Jini, D.; Joseph, B. Physiological Mechanism of Salicylic Acid for Alleviation of Salt Stress in Rice. Rice Sci. 2017, 24, 97–108. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. How Can Salicylic Acid and Jasmonic Acid Mitigate Salt Toxicity in Soybean Plants? Ecotoxicol. Environ. Saf. 2018, 154, 1010–1016. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Siddiqui, M.N.; Sohag, A.A.M.; Sakil, M.A.; Rahman, M.M.; Polash, M.A.S.; Mostofa, M.G.; Tran, L.-S.P. Salicylic Acid-Mediated Enhancement of Photosynthesis Attributes and Antioxidant Capacity Contributes to Yield Improvement of Maize Plants Under Salt Stress. J. Plant Growth Regul. 2018, 37, 1318–1330. [Google Scholar] [CrossRef]
- Naeem, M.; Basit, A.; Ahmad, I.; Mohamed, H.I.; Wasila, H. Effect of Salicylic Acid and Salinity Stress on the Performance of Tomato Plants. Gesunde Pflanz. 2020, 72, 393–402. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Ranjbar, G.; Hasanuzzaman, M.; Khanna, K.; Bhardwaj, R.; Ahmad, P. Salicylic Acid-Mediated Regulation of Morpho-Physiological and Yield Attributes of Wheat and Barley Plants in Deferring Salinity Stress. J. Plant Growth Regul. 2022, 41, 1291–1303. [Google Scholar] [CrossRef]
- Torun, H.; Novák, O.; Mikulík, J.; Strnad, M.; Ayaz, F.A. The Effects of Exogenous Salicylic Acid on Endogenous Phytohormone Status in Hordeum vulgare L. under Salt Stress. Plants 2022, 11, 618. [Google Scholar] [CrossRef]
- Rady, M.M.; Mohamed, G.F. Modulation of Salt Stress Effects on the Growth, Physio-Chemical Attributes and Yields of Phaseolus vulgaris L. Plants by the Combined Application of Salicylic Acid and Moringa oleifera Leaf Extract. Sci. Hortic. 2015, 193, 105–113. [Google Scholar] [CrossRef]
- Rady, M. Integrated Application of Salicylic Acid and Moringa Oleifera Leaf Extract Alleviates the Salt-Induced Adverse Effects in Common Bean Plants. Int. J. Agric. Technol. 2015, 11, 1613–1633. [Google Scholar]
- Dawood, M.F.A.; Zaid, A.; Latef, A.A.H.A. Salicylic Acid Spraying-Induced Resilience Strategies Against the Damaging Impacts of Drought and/or Salinity Stress in Two Varieties of Vicia faba L. Seedlings. J. Plant Growth Regul. 2022, 41, 1919–1942. [Google Scholar] [CrossRef]
- El-Beltagi, H.; Ahmed, S.; Namich, A.; Abdel-Sattar, R. Effect of Salicylic Acid and Potassium Citrate on Cotton Plant under Salt Stress. Fresenius Environ. Bull. 2017, 26, 1091–1100. [Google Scholar]
- Ilyas, M.; Maqsood, M.F.; Shahbaz, M.; Zulfiqar, U.; Ahmad, K.; Naz, N.; Ali, M.F.; Ahmad, M.; Ali, Q.; Yong, J.W.H.; et al. Alleviating Salinity Stress in Canola (Brassica napus L.) through Exogenous Application of Salicylic Acid. BMC Plant Biol. 2024, 24, 611. [Google Scholar] [CrossRef] [PubMed]
- Linić, I.; Mlinarić, S.; Brkljačić, L.; Pavlović, I.; Smolko, A.; Salopek-Sondi, B. Ferulic Acid and Salicylic Acid Foliar Treatments Reduce Short-Term Salt Stress in Chinese Cabbage by Increasing Phenolic Compounds Accumulation and Photosynthetic Performance. Plants 2021, 10, 2346. [Google Scholar] [CrossRef]
- Dong, Y.J.; Wang, Z.L.; Zhang, J.W.; Liu, S.; He, Z.L.; He, M.R. Interaction Effects of Nitric Oxideand Salicylic Acid in Alleviating Salt Stress of Gossypium hirsutum L. J. Soil Sci. Plant Nutr. 2015, 15, 561–573. [Google Scholar] [CrossRef]
- Keya, S.S.; Mostofa, M.G.; Rahman, M.M.; Das, A.K.; Sultana, S.; Ghosh, P.K.; Anik, T.R.; Ahsan, S.M.; Rahman, M.A.; Jahan, N.; et al. Salicylic Acid Application Improves Photosynthetic Performance and Biochemical Responses to Mitigate Saline Stress in Cotton. J. Plant Growth Regul. 2023, 42, 5881–5894. [Google Scholar] [CrossRef]
- El-Taher, A.M.; Abd El-Raouf, H.S.; Osman, N.A.; Azoz, S.N.; Omar, M.A.; Elkelish, A.; Abd El-Hady, M.A.M. Effect of Salt Stress and Foliar Application of Salicylic Acid on Morphological, Biochemical, Anatomical, and Productivity Characteristics of Cowpea (Vigna unguiculata L.) Plants. Plants 2022, 11, 115. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.; Shim, I.-S. Exogenous Salicylic Acid Alleviates Salt-Stress Damage in Cucumber under Moderate Nitrogen Conditions by Controlling Endogenous Salicylic Acid Levels. Hortic. Environ. Biotechnol. 2017, 58, 247–253. [Google Scholar] [CrossRef]
- Omidi, M.; Khandan-Mirkohi, A.; Kafi, M.; Zamani, Z.; Ajdanian, L.; Babaei, M. Biochemical and Molecular Responses of Rosa Damascena Mill. Cv. Kashan to Salicylic Acid under Salinity Stress. BMC Plant Biol. 2022, 22, 373. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic Acid Alleviates the Adverse Effects of Salt Stress on Dianthus superbus (Caryophyllaceae) by Activating Photosynthesis, Protecting Morphological Structure, and Enhancing the Antioxidant System. Front. Plant Sci. 2017, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Mady, E.; Abd El-Wahed, A.H.M.; Awad, A.H.; Asar, T.O.; Al-Farga, A.; Abd El-Raouf, H.S.; Randhir, R.; Alnuzaili, E.S.; El-Taher, A.M.; Randhir, T.O.; et al. Evaluation of Salicylic Acid Effects on Growth, Biochemical, Yield, and Anatomical Characteristics of Eggplant (Solanum melongena L.) Plants under Salt Stress Conditions. Agronomy 2023, 13, 2213. [Google Scholar] [CrossRef]
- Batista, V.C.V.; Pereira, I.M.C.; de Oliveira Paula-Marinho, S.; Canuto, K.M.; Pereira, R.D.C.A.; Rodrigues, T.H.S.; de Menezes Daloso, D.; Gomes-Filho, E.; de Carvalho, H.H. Salicylic Acid Modulates Primary and Volatile Metabolites to Alleviate Salt Stress-Induced Photosynthesis Impairment on Medicinal Plant Egletes viscosa. Environ. Exp. Bot. 2019, 167, 103870. [Google Scholar] [CrossRef]
- Husen, A.; Iqbal, M.; Sohrab, S.S.; Ansari, M.K.A. Salicylic Acid Alleviates Salinity-Caused Damage to Foliar Functions, Plant Growth and Antioxidant System in Ethiopian Mustard (Brassica carinata A. Br.). Agric. Food Secur. 2018, 7, 44. [Google Scholar] [CrossRef]
- Abdelhameed, R.E.; Abdel Latef, A.A.H.; Shehata, R.S. Physiological Responses of Salinized Fenugreek (Trigonellafoenum-graecum L.) Plants to Foliar Application of Salicylic Acid. Plants 2021, 10, 657. [Google Scholar] [CrossRef]
- Mallahi, T.; Saharkhiz, M.J.; Javanmardi, J. Salicylic Acid Changes Morpho-Physiological Attributes of Feverfew (Tanacetum parthenium L.) under Salinity Stress. Acta Ecol. Sin. 2018, 38, 351–355. [Google Scholar] [CrossRef]
- Liu, X.; Ma, D.; Zhang, Z.; Wang, S.; Du, S.; Deng, X.; Yin, L. Plant Lipid Remodeling in Response to Abiotic Stresses. Environ. Exp. Bot. 2019, 165, 174–184. [Google Scholar] [CrossRef]
- Manzoor, K.; Ilyas, N.; Batool, N.; Ahmad, B.; Arshad, M. Effect of Salicylic Acid on the Growth and Physiological Characteristics of Maize under Stress Conditions. J. Chem. Soc. Pak. 2015, 37, 588. [Google Scholar]
- El-Katony, T.M.; El-Bastawisy, Z.M.; El-Ghareeb, S.S. Timing of Salicylic Acid Application Affects the Response of Maize (Zea mays L.) Hybrids to Salinity Stress. Heliyon 2019, 5, e01547. [Google Scholar] [CrossRef]
- Elhakem, A.H. Salicylic Acid Ameliorates Salinity Tolerance in Maize by Regulation of Phytohormones and Osmolytes. Plant Soil Environ. 2020, 66, 533–541. [Google Scholar] [CrossRef]
- Ali, E.; Hussain, S.; Jalal, F.; Khan, M.A.; Imtiaz, M.; Said, F.; Ismail, M.; Khan, S.; Ali, H.M.; Hatamleh, A.A.; et al. Salicylic Acid-Mitigates Abiotic Stress Tolerance via Altering Defense Mechanisms in Brassica napus (L.). Front. Plant Sci. 2023, 14, 1187260. [Google Scholar] [CrossRef] [PubMed]
- Barwal, S.K.; Shah, S.H.; Pawar, A.; Siddiqui, M.H.; Agnihotri, R.K.; Vimala, Y.; Wani, S.H. Mechanistic insights of salicylic acid-mediated salt stress tolerance in Zea mays L. seedlings. Heliyon 2024, 10, e34486. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Mao, J.; Wu, T.; Xiong, T.; Huang, Q.; Wu, H.; Hu, G. Transcriptomic Analysis of Salicylic Acid Promoting Seed Germination of Melon under Salt Stress. Horticulturae 2023, 9, 375. [Google Scholar] [CrossRef]
- Azad, N.; Rezayian, M.; Hassanpour, H.; Niknam, V.; Ebrahimzadeh, H. Physiological Mechanism of Salicylic Acid in Mentha pulegium L. under Salinity and Drought Stress. Braz. J. Bot. 2021, 44, 359–369. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Lotfi, R. The Impact of Salicylic Acid and Silicon on Chlorophyll a Fluorescence in Mung Bean under Salt Stress. Russ. J. Plant Physiol. 2015, 62, 611–616. [Google Scholar] [CrossRef]
- Lotfi, R.; Ghassemi-Golezani, K.; Pessarakli, M. Salicylic Acid Regulates Photosynthetic Electron Transfer and Stomatal Conductance of Mung Bean (Vigna radiata L.) under Salinity Stress. Biocatal. Agric. Biotechnol. 2020, 26, 101635. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic Acid Supplementation Improves Photosynthesis and Growth in Mustard through Changes in Proline Accumulation and Ethylene Formation under Drought Stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Islam, S.; Mohammad, F.; Siddiqui, M.H.; Kalaji, H.M. Salicylic Acid and Trehalose Attenuate Salt Toxicity in Brassica juncea L. by Activating the Stress Defense Mechanism. Environ. Pollut. 2023, 326, 121467. [Google Scholar] [CrossRef]
- Yan, Y.; Pan, C.; Du, Y.; Li, D.; Liu, W. Exogenous Salicylic Acid Regulates Reactive Oxygen Species Metabolism and Ascorbate–Glutathione Cycle in Nitraria tangutorum Bobr. under Salinity Stress. Physiol. Mol. Biol. Plants 2018, 24, 577–589. [Google Scholar] [CrossRef]
- Esan, A.M.; Masisi, K.; Dada, F.A.; Olaiya, C.O. Comparative Effects of Indole Acetic Acid and Salicylic Acid on Oxidative Stress Marker and Antioxidant Potential of Okra (Abelmoschus esculentus) Fruit under Salinity Stress. Sci. Hortic. 2017, 216, 278–283. [Google Scholar] [CrossRef]
- Torres Mendonça, A.J.; Silva, A.A.R.D.; Lima, G.S.D.; Soares, L.A.D.A.; Nunes Oliveira, V.K.; Gheyi, H.R.; Lacerda, C.F.D.; Azevedo, C.A.V.D.; Lima, V.L.A.D.; Fernandes, P.D. Salicylic Acid Modulates Okra Tolerance to Salt Stress in Hydroponic System. Agriculture 2022, 12, 1687. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Hajilou, J.; Tabatabaei, S.J. Photosynthetic and Growth Responses of Olive to Proline and Salicylic Acid under Salinity Condition. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 579–585. [Google Scholar] [CrossRef]
- Yadu, S.; Dewangan, T.L.; Chandrakar, V.; Keshavkant, S. Imperative Roles of Salicylic Acid and Nitric Oxide in Improving Salinity Tolerance in Pisum sativum L. Physiol. Mol. Biol. Plants 2017, 23, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.; Kumar, A.; Yadav, R.K.; Yadav, G.; Kumar, R.; Kushwaha, M. Salicylic Acid and Thiourea Mitigate the Salinity and Drought Stress on Physiological Traits Governing Yield in Pearl Millet-Wheat. Saudi J. Biol. Sci. 2020, 27, 2010–2017. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhadi, N. The Efficacy of Salicylic Acid Levels on Photosynthetic Activity, Growth, and Essential Oil Content and Composition of Pennyroyal Plants Under Salt Stress. J. Plant Growth Regul. 2022, 41, 1953–1965. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The Role of Endogenous Nitric Oxide in Salicylic Acid-Induced up-Regulation of Ascorbate-Glutathione Cycle Involved in Salinity Tolerance of Pepper (Capsicum annuum L.) Plants. Plant Physiol. Biochem. 2020, 147, 10–20. [Google Scholar] [CrossRef]
- Khalil, H.A.; El-Ansary, D.O.; Ahmed, Z.F.R. Mitigation of Salinity Stress on Pomegranate (Punica granatum L. Cv. Wonderful) Plant Using Salicylic Acid Foliar Spray. Horticulturae 2022, 8, 375. [Google Scholar] [CrossRef]
- Bukhat, S.; Manzoor, H.; Athar, H.-R.; Zafar, Z.U.; Azeem, F.; Rasul, S. Salicylic Acid Induced Photosynthetic Adaptability of Raphanus sativus to Salt Stress Is Associated with Antioxidant Capacity. J. Plant Growth Regul. 2020, 39, 809–822. [Google Scholar] [CrossRef]
- Kim, Y.; Mun, B.-G.; Khan, A.L.; Waqas, M.; Kim, H.-H.; Shahzad, R.; Imran, M.; Yun, B.-W.; Lee, I.-J. Regulation of Reactive Oxygen and Nitrogen Species by Salicylic Acid in Rice Plants under Salinity Stress Conditions. PLoS ONE 2018, 13, e0192650. [Google Scholar] [CrossRef]
- Khan, M.S.; Akther, T.; Mubarak Ali, D.; Hemalatha, S. An Investigation on the Role of Salicylic Acid Alleviate the Saline Stress in Rice Crop (Oryza Sativa (L)). Biocatal. Agric. Biotechnol. 2019, 18, 101027. [Google Scholar] [CrossRef]
- Shan, L.; Xu, Y.; Wu, D.; Hu, J.; Yu, T.; Dang, C.; Fang, Y.; Zhang, X.; Tian, Q.; Xue, D. Effects of Salicylic Acid on Growth, Physiology, and Gene Expression in Rice Seedlings under Salt and Drought Stress. Plant Stress 2024, 11, 100413. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Elansary, H.O.; El-Shanhorey, N.A.; Abdel-Hamid, A.M.E.; Ali, H.M.; Elshikh, M.S. Salicylic Acid-Regulated Antioxidant Mechanisms and Gene Expression Enhance Rosemary Performance under Saline Conditions. Front. Physiol. 2017, 8, 716. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi-Golezani, K.; Hosseinzadeh-Mahootchi, A. Improving Physiological Performance of Safflower under Salt Stress by Application of Salicylic Acid and Jasmonic Acid. WALIA J. 2015, 31, 104–109. [Google Scholar]
- Golkar, P.; Taghizadeh, M.; Yousefian, Z. The Effects of Chitosan and Salicylic Acid on Elicitation of Secondary Metabolites and Antioxidant Activity of Safflower under in Vitro Salinity Stress. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 137, 575–585. [Google Scholar] [CrossRef]
- Shaki, F.; Maboud, H.E.; Niknam, V. Growth Enhancement and Salt Tolerance of Safflower (Carthamus tinctorius L.), by Salicylic Acid. Curr. Plant Biol. 2018, 13, 16–22. [Google Scholar] [CrossRef]
- Shaki, F.; Maboud, H.E.; Niknam, V. Effects of Salicylic Acid on Hormonal Cross Talk, Fatty Acids Profile, and Ions Homeostasis from Salt-Stressed Safflower. J. Plant Interact. 2019, 14, 340–346. [Google Scholar] [CrossRef]
- Es-sbihi, F.Z.; Hazzoumi, Z.; Aasfar, A.; Amrani Joutei, K. Improving Salinity Tolerance in Salvia officinalis L. by Foliar Application of Salicylic Acid. Chem. Biol. Technol. Agric. 2021, 8, 25. [Google Scholar] [CrossRef]
- Jangra, M.; Devi, S.; Satpal; Kumar, N.; Goyal, V.; Mehrotra, S. Amelioration Effect of Salicylic Acid Under Salt Stress in Sorghum bicolor L. Appl. Biochem. Biotechnol. 2022, 194, 4400–4423. [Google Scholar] [CrossRef]
- Silva, A.A.R.D.; Lima, G.S.D.; Azevedo, C.A.V.D.; Gheyi, H.R.; Souza, A.R.D.; Fernandes, P.D. Salicylic Acid Relieves the Effect of Saline Stress on Soursop Morphysiology. Ciência E Agrotecnologia 2021, 45, e007021. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Changes in Oil Accumulation and Fatty Acid Composition of Soybean Seeds under Salt Stress in Response to Salicylic Acid and Jasmonic Acid. Russ. J. Plant Physiol. 2018, 65, 229–236. [Google Scholar] [CrossRef]
- Faghih, S.; Ghobadi, C.; Zarei, A. Response of Strawberry Plant Cv. ‘Camarosa’ to Salicylic Acid and Methyl Jasmonate Application Under Salt Stress Condition. J. Plant Growth Regul. 2017, 36, 651–659. [Google Scholar] [CrossRef]
- Roshdy, A.E.-D.; Alebidi, A.; Almutairi, K.; Al-Obeed, R.; Elsabagh, A. The Effect of Salicylic Acid on the Performances of Salt Stressed Strawberry Plants, Enzymes Activity, and Salt Tolerance Index. Agronomy 2021, 11, 775. [Google Scholar] [CrossRef]
- Noreen, S.; Siddiq, A.; Hussain, K.; Ahmad, S.; Hasanuzzama, M. Foliar Application of Salicylic Acid with Salinity Stress on Physiological and Biochemical Attributes of Sunflower (Helianthus annuus L.) Crop. Acta Sci. Polonorum. Hortorum Cultus 2017, 16, 57–74. [Google Scholar]
- Horváth, E.; Csiszár, J.; Gallé, Á.; Poór, P.; Szepesi, Á.; Tari, I. Hardening with Salicylic Acid Induces Concentration-Dependent Changes in Abscisic Acid Biosynthesis of Tomato under Salt Stress. J. Plant Physiol. 2015, 183, 54–63. [Google Scholar] [CrossRef]
- Szepesi, Á. The Alleviation of the Adverse Effects of Salt Stress in the Tomato Plant by Salicylic Acid Shows A Time- and Organ-Specific Antioxidant Response. Acta Biol. Cracoviensia. Ser. Bot. 2015, 57, 21–30. [Google Scholar]
- Souri, M.K.; Tohidloo, G. Effectiveness of Different Methods of Salicylic Acid Application on Growth Characteristics of Tomato Seedlings under Salinity. Chem. Biol. Technol. Agric. 2019, 6, 26. [Google Scholar] [CrossRef]
- Talaat, N.B. Polyamine and Nitrogen Metabolism Regulation by Melatonin and Salicylic Acid Combined Treatment as a Repressor for Salt Toxicity in Wheat (Triticum aestivum L.) Plants. Plant Growth Regul. 2021, 95, 315–329. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Synergistic Effects of Salicylic Acid and Melatonin on Modulating Ion Homeostasis in Salt-Stressed Wheat (Triticum aestivum L.) Plants by Enhancing Root H+-Pump Activity. Plants 2022, 11, 416. [Google Scholar] [CrossRef]
- Alam, A.; Ullah, H.; Thuenprom, N.; Tisarum, R.; Cha-um, S.; Datta, A. Seed Priming with Salicylic Acid Enhances Growth, Physiological Traits, Fruit Yield, and Quality Parameters of Cantaloupe under Water-Deficit Stress. S. Afr. J. Bot. 2022, 150, 1–12. [Google Scholar] [CrossRef]
- Torun, H.; Novák, O.; Mikulík, J.; Pěnčík, A.; Strnad, M.; Ayaz, F.A. Timing-Dependent Effects of Salicylic Acid Treatment on Phytohormonal Changes, ROS Regulation, and Antioxidant Defense in Salinized Barley (Hordeum vulgare L.). Sci. Rep. 2020, 10, 13886. [Google Scholar] [CrossRef]
- Loutfy, N.; Hassanein, A.M.; Inouhe, M.; Salem, J.M. Biological Aspects and Proline Metabolism Genes Influenced by Polyethylene Glycol and Salicylic Acid in Two Wheat Cultivars. Egypt. J. Bot. 2022, 62, 671–685. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Brestič, M.; Afrin, S.; Sakil, M.A.; Hossain, M.T.; Hossain, M.A.; Hossain, M.A. Exogenous Salicylic Acid and Hydrogen Peroxide Attenuate Drought Stress in Rice. Plant Soil Environ. 2020, 66, 7–13. [Google Scholar] [CrossRef]
- Arruda, T.F.D.L.; Lima, G.S.D.; Silva, A.A.R.D.; Azevedo, C.A.V.D.; Souza, A.R.D.; Soares, L.A.D.A.; Saboya, L.M.F. Salicylic Acid as a Salt Stress Mitigator on Chlorophyll Fluorescence, Photosynthetic Pigments, and Growth of Precocious-Dwarf Cashew in the Post-Grafting Phase. Plants 2023, 12, 2783. [Google Scholar] [CrossRef]
- Afzal, S.; Chaudhary, N.; Singh, N.K. Role of Soluble Sugars in Metabolism and Sensing Under Abiotic Stress. In Plant Growth Regulators; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Altaf, M.M.; Diao, X.P.; Wang, H.; Khan, L.U.; Rehman, A.U.; Shakoor, A.; Farooq, T.H. Salicylic Acid Induces Vanadium Stress Tolerance in Rice by Regulating the AsA-GSH Cycle and Glyoxalase System. J. Soil Sci. Plant Nutr. 2022, 22, 1983–1999. [Google Scholar] [CrossRef]
- Jia, C.; Guo, B.; Wang, B.; Li, X.; Yang, T.; Li, N.; Yu, Q. Integrated Metabolomic and Transcriptomic Analysis Reveals the Role of Phenylpropanoid Biosynthesis Pathway in Tomato Roots during Salt Stress. Front. Plant Sci. 2022, 13, 1023696. [Google Scholar] [CrossRef]
- Wang, F.; Xu, H.; He, W.; Lu, Y.; Luo, H.; Guo, Q.; Zhang, Z. Exogenous Salicylic Acid Promotes Carotenoid Accumulation and Antioxidant Capacity in Germinated Maize Kernels by Regulating Carotenoid Biosynthetic Pathway. Food Biosci. 2024, 59, 103990. [Google Scholar] [CrossRef]
- Kobyletska, M.; Kavulych, Y. The Effect of Salicylic Acid on the Content of Ascorbic Acid and Phenolic Compounds in Wheat Plants. Біoлoгічні студії/Stud. Biol. 2024, 18, 125–137. [Google Scholar] [CrossRef]
- Latif, F.; Ullah, F.; Mehmood, S.; Khattak, A.; Khan, A.U.; Khan, S.; Husain, I. Effects of Salicylic Acid on Growth and Accumulation of Phenolics in Zea mays L. under Drought Stress. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2016, 66, 325–332. [Google Scholar]
- Li, Q.; Wang, G.; Guan, C.; Yang, D.; Wang, Y.; Zhang, Y.; Ji, J.; Jin, C.; An, T. Overexpression of LcSABP, an Orthologous Gene for Salicylic Acid Binding Protein 2, Enhances Drought Stress Tolerance in Transgenic Tobacco. Front. Plant Sci. 2019, 10, 200. [Google Scholar] [CrossRef]
- La, V.H.; Lee, B.-R.; Zhang, Q.; Park, S.-H.; Islam, M.T.; Kim, T.-H. Salicylic Acid Improves Drought-Stress Tolerance by Regulating the Redox Status and Proline Metabolism in Brassica Rapa. Hortic. Environ. Biotechnol 2019, 60, 31–40. [Google Scholar] [CrossRef]
- Estaji, A.; Niknam, F. Foliar Salicylic Acid Spraying Effect’ on Growth, Seed Oil Content, and Physiology of Drought-Stressed Silybum marianum L. Plant. Agric. Water Manag. 2020, 234, 106116. [Google Scholar] [CrossRef]
- Khalvandi, M.; Siosemardeh, A.; Roohi, E.; Keramati, S. Salicylic Acid Alleviated the Effect of Drought Stress on Photosynthetic Characteristics and Leaf Protein Pattern in Winter Wheat. Heliyon 2021, 7, e05908. [Google Scholar] [CrossRef] [PubMed]
- Aires, E.S.; Ferraz, A.K.L.; Carvalho, B.L.; Teixeira, F.P.; Putti, F.F.; de Souza, E.P.; Rodrigues, J.D.; Ono, E.O. Foliar Application of Salicylic Acid to Mitigate Water Stress in Tomato. Plants 2022, 11, 1775. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; da Silva Júnior, G.B.; Bonifácio, A.; Dutra, A.F.; de Mello Prado, R.; de Alcântara Neto, F.; Zuffo, A.M.; Melo, R.S.; de Sousa Pereira, T.L.; de Sousa, R.S. Exogenous Salicylic Acid Alleviates Water Stress in Watermelon Plants. Ann. Appl. Biol. 2023, 182, 121–130. [Google Scholar] [CrossRef]
- Osama, S.; El Sherei, M.; Al-Mahdy, D.A.; Bishr, M.; Salama, O. Effect of Salicylic Acid Foliar Spraying on Growth Parameters, γ-Pyrones, Phenolic Content and Radical Scavenging Activity of Drought Stressed Ammi visnaga L. Plant. Ind. Crops Prod. 2019, 134, 1–10. [Google Scholar] [CrossRef]
- González-Villagra, J.; Reyes-Díaz, M.M.; Tighe-Neira, R.; Inostroza-Blancheteau, C.; Escobar, A.L.; Bravo, L.A. Salicylic Acid Improves Antioxidant Defense System and Photosynthetic Performance in Aristotelia chilensis Plants Subjected to Moderate Drought Stress. Plants 2022, 11, 639. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.-S.E.; Abu-Elsaoud, A.M.; Hafez, Y.M. Exogenous Application of Proline and Salicylic Acid Can Mitigate the Injurious Impacts of Drought Stress on Barley Plants Associated with Physiological and Histological Characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Peng, Y.; Huang, B. Metabolic Pathways Regulated by Abscisic Acid, Salicylic Acid and γ-Aminobutyric Acid in Association with Improved Drought Tolerance in Creeping Bentgrass (Agrostis Stolonifera). Physiol. Plant. 2017, 159, 42–58. [Google Scholar] [CrossRef]
- Abbaspour, N.; Babaee, L. Effect of Salicylic Acid Application on Oxidative Damage and Antioxidant Activity of Grape (Vitis vinifera L.) under Drought Stress Condition. Int. J. Hortic. Sci. Technol. 2017, 4, 29–50. [Google Scholar] [CrossRef]
- Safari, M.; Mousavi-Fard, S.; Rezaei Nejad, A.; Sorkheh, K.; Sofo, A. Exogenous Salicylic Acid Positively Affects Morpho-Physiological and Molecular Responses of Impatiens walleriana Plants Grown under Drought Stress. Int. J. Environ. Sci. Technol. 2022, 19, 969–984. [Google Scholar] [CrossRef]
- Dianat, M.; Saharkhiz, M.J.; Tavassolian, I. Salicylic Acid Mitigates Drought Stress in Lippia citriodora L.: Effects on Biochemical Traits and Essential Oil Yield. Biocatal. Agric. Biotechnol. 2016, 8, 286–293. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.-S.M.S.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of Salicylic Acid, Zinc and Glycine Betaine on Morpho-Physiological Growth and Yield of Maize under Drought Stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Fatma, M.; Gautam, H.; Sehar, Z.; Rasheed, F.; Khan, M.I.R.; Sofo, A.; Khan, N.A. Salicylic Acid Increases Photosynthesis of Drought Grown Mustard Plants Effectively with Sufficient-N via Regulation of Ethylene, Abscisic Acid, and Nitrogen-Use Efficiency. J. Plant Growth Regul. 2022, 41, 1966–1977. [Google Scholar] [CrossRef]
- Semida, W.M.; Abd El-Mageed, T.A.; Mohamed, S.E.; El-Sawah, N.A. Combined Effect of Deficit Irrigation and Foliar-Applied Salicylic Acid on Physiological Responses, Yield, and Water-Use Efficiency of Onion Plants in Saline Calcareous Soil. Arch. Agron. Soil Sci. 2017, 63, 1227–1239. [Google Scholar] [CrossRef]
- Biareh, V.; Shekari, F.; Sayfzadeh, S.; Zakerin, H.; Hadidi, E.; Beltrão, J.G.T.; Mastinu, A. Physiological and Qualitative Response of Cucurbita pepo L. to Salicylic Acid under Controlled Water Stress Conditions. Horticulturae 2022, 8, 79. [Google Scholar] [CrossRef]
- Saheri, F.; Barzin, G.; Pishkar, L.; Boojar, M.M.A.; Babaeekhou, L. Foliar Spray of Salicylic Acid Induces Physiological and Biochemical Changes in Purslane (Portulaca oleracea L.) under Drought Stress. Biologia 2020, 75, 2189–2200. [Google Scholar] [CrossRef]
- Henschel, J.M.; Dantas, E.F.O.; de Azevedo Soares, V.; Dos Santos, S.K.; Dos Santos, L.W.O.; Dias, T.J.; Batista, D.S. Salicylic Acid Mitigates the Effects of Mild Drought Stress on Radish (Raphanus sativus) Growth. Funct. Plant Biol. 2022, 49, 822–831. [Google Scholar] [CrossRef]
- Abbaszadeh, B.; Layeghhaghighi, M.; Azimi, R.; Hadi, N. Improving Water Use Efficiency through Drought Stress and Using Salicylic Acid for Proper Production of Rosmarinus officinalis L. Ind. Crops Prod. 2020, 144, 111893. [Google Scholar] [CrossRef]
- Attia, E.Z.; Abd El-Baky, R.M.; Desoukey, S.Y.; El Hakeem Mohamed, M.A.; Bishr, M.M.; Kamel, M.S. Chemical Composition and Antimicrobial Activities of Essential Oils of Ruta graveolens Plants Treated with Salicylic Acid under Drought Stress Conditions. Future J. Pharm. Sci. 2018, 4, 254–264. [Google Scholar] [CrossRef]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Improvement in Drought Stress Tolerance of Safflower during Vegetative Growth by Exogenous Application of Salicylic Acid and Sodium Nitroprusside. Ind. Crops Prod. 2019, 134, 168–176. [Google Scholar] [CrossRef]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Effect of Salicylic Acid and Sodium Nitroprusside on Growth Parameters, Photosynthetic Pigments and Secondary Metabolites of Safflower under Drought Stress. Sci. Hortic. 2020, 259, 108823. [Google Scholar] [CrossRef]
- Hussein, Y.; Amin, G.; Azab, A.; Gahin, H. Induction of Drought Stress Resistance in Sesame (Sesamum indicum L.) Plant by Salicylic Acid and Kinetin. J. Plant Sci. 2015, 10, 128–141. [Google Scholar] [CrossRef]
- Yousefzadeh Najafabadi, M.; Ehsanzadeh, P. Photosynthetic and Antioxidative Upregulation in Drought-Stressed Sesame (Sesamum indicum L.) Subjected to Foliar-Applied Salicylic Acid. Photosynthetica 2017, 55, 611–622. [Google Scholar] [CrossRef]
- Pourghasemian, N.; Moradi, R.; Naghizadeh, M.; Landberg, T. Mitigating Drought Stress in Sesame by Foliar Application of Salicylic Acid, Beeswax Waste and Licorice Extract. Agric. Water Manag. 2020, 231, 105997. [Google Scholar] [CrossRef]
- Yousefvand, P.; Sohrabi, Y.; Heidari, G.; Weisany, W.; Mastinu, A. Salicylic Acid Stimulates Defense Systems in Allium Hirtifolium Grown under Water Deficit Stress. Molecules 2022, 27, 3083. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Semida, W.M.; Mohamed, G.F.; Rady, M.M. Combined Effect of Foliar-Applied Salicylic Acid and Deficit Irrigation on Physiological–Anatomical Responses, and Yield of Squash Plants under Saline Soil. S. Afr. J. Bot. 2016, 106, 8–16. [Google Scholar] [CrossRef]
- Ghaderi, N.; Normohammadi, S.; Javadi, T. Morpho-Physiological Responses of Strawberry (Fragaria × Ananassa) to Exogenous Salicylic Acid Application under Drought Stress. J. Agric. Sci. Technol. 2015, 17, 167–178. [Google Scholar]
- El-Bially, M.E.; Saudy, H.S.; Hashem, F.A.; El-Gabry, Y.A.; Shahin, M.G. Salicylic Acid as a Tolerance Inducer of Drought Stress on Sunflower Grown in Sandy Soil. Gesunde Pflanz. 2022, 74, 603–613. [Google Scholar] [CrossRef]
- Damalas, C.A. Improving Drought Tolerance in Sweet Basil (Ocimum basilicum) with Salicylic Acid. Sci. Hortic. 2019, 246, 360–365. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Chen, J.; Finnegan, P.M.; Younis, A.; Nafees, M.; Zorrig, W.; Hamed, K.B. Application of Trehalose and Salicylic Acid Mitigates Drought Stress in Sweet Basil and Improves Plant Growth. Plants 2021, 10, 1078. [Google Scholar] [CrossRef]
- Khalil, N.; Fekry, M.; Bishr, M.; El-Zalabani, S.; Salama, O. Foliar Spraying of Salicylic Acid Induced Accumulation of Phenolics, Increased Radical Scavenging Activity and Modified the Composition of the Essential Oil of Water Stressed Thymus vulgaris L. Plant Physiol. Biochem. 2018, 123, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Amirikia, F.; Ghorbanpour, M.; Fatehi, F.; Hashempour, H. Salicylic Acid Induced Changes in Physiological Traits and Essential Oil Constituents in Different Ecotypes of Thymus kotschyanus and Thymus vulgaris under Well-Watered and Water Stress Conditions. Ind. Crops Prod. 2019, 129, 561–574. [Google Scholar] [CrossRef]
- Lobato, A.K.D.S.; Barbosa, M.A.M.; Alsahli, A.A.; Lima, E.J.A.; Silva, B.R.S.D. Exogenous Salicylic Acid Alleviates the Negative Impacts on Production Components, Biomass and Gas Exchange in Tomato Plants under Water Deficit Improving Redox Status and Anatomical Responses. Physiol. Plant. 2021, 172, 869–884. [Google Scholar] [CrossRef]
- Hassanein, R.; Amin, A.A.E.-S.; Rashad, E.M.; Ali, H. Effect of thiourea and salicylic acid on antioxidant defense of wheat plants under drought stress. Int. J. ChemTech Res. 2015, 7, 346–354. [Google Scholar]
- Yavaş, İ.; Ünay, A. Effects of Zinc and Salicylic Acid on Wheat under Drought Stress. JAPS J. Anim. Plant Sci. 2016, 26, 1012–1018. [Google Scholar]
- Ilyas, N.; Gull, R.; Mazhar, R.; Saeed, M.; Kanwal, S.; Shabir, S.; Bibi, F. Influence of Salicylic Acid and Jasmonic Acid on Wheat Under Drought Stress. Commun. Soil Sci. Plant Anal. 2017, 48, 2715–2723. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, S.K.; Majumder, B.; Maurya, V.K.; Deeba, F.; Alam, A.; Pandey, V. Salicylic Acid Mediated Growth, Physiological and Proteomic Responses in Two Wheat Varieties under Drought Stress. J. Proteom. 2017, 163, 28–51. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Ashraf, M.; Arvin, M.J. Alleviation of Field Water Stress in Wheat Cultivars by Using Silicon and Salicylic Acid Applied Separately or in Combination. Crop Pasture Sci. 2019, 70, 36–43. [Google Scholar] [CrossRef]
- Parveen, A.; Arslan Ashraf, M.; Hussain, I.; Perveen, S.; Rasheed, R.; Mahmood, Q.; Hussain, S.; Ditta, A.; Hashem, A.; Al-Arjani, A.-B.F.; et al. Promotion of Growth and Physiological Characteristics in Water-Stressed Triticum Aestivum in Relation to Foliar-Application of Salicylic Acid. Water 2021, 13, 1316. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, Y.; Wang, F.; Ye, Y.; Zhu, C. Role of Salicylic Acid in Resistance to Cadmium Stress in Plants. Plant Cell Rep. 2016, 35, 719–731. [Google Scholar] [CrossRef]
- Guo, B.; Liu, C.; Liang, Y.; Li, N.; Fu, Q. Salicylic Acid Signals Plant Defence against Cadmium Toxicity. Int. J. Mol. Sci. 2019, 20, 2960. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Handa, N.; Kaur, R.; Kumar, V.; Khanna, K.; Bakshi, P.; Singh, R.; Arora, S.; Kaur, R.; Bhardwaj, R. Role of Salicylic Acid in Heavy Metal Stress Tolerance: Insight into Underlying Mechanism. In Salicylic Acid: A Multifaceted Hormone; Nazar, R., Iqbal, N., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 123–144. ISBN 978-981-10-6068-7. [Google Scholar]
- Singh, A.P.; Dixit, G.; Mishra, S.; Dwivedi, S.; Tiwari, M.; Mallick, S.; Pandey, V.; Trivedi, P.K.; Chakrabarty, D.; Tripathi, R.D. Salicylic Acid Modulates Arsenic Toxicity by Reducing Its Root to Shoot Translocation in Rice (Oryza sativa L.). Front. Plant Sci. 2015, 6, 340. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Daud, M.K.; Ullah, N.; Ali, S.; Khan, M.; Malik, Z.; Zhu, S.J. Pretreatment with Salicylic Acid and Ascorbic Acid Significantly Mitigate Oxidative Stress Induced by Copper in Cotton Genotypes. Environ. Sci. Pollut. Res. 2015, 22, 9922–9931. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Allagulova, C.R.; Maslennikova, D.R.; Klyuchnikova, E.O.; Avalbaev, A.M.; Bezrukova, M.V. Salicylic Acid-Induced Protection against Cadmium Toxicity in Wheat Plants. Environ. Exp. Bot. 2016, 122, 19–28. [Google Scholar] [CrossRef]
- Saidi, I.; Yousfi, N.; Borgi, M.A. Salicylic Acid Improves the Antioxidant Ability against Arsenic-Induced Oxidative Stress in Sunflower (Helianthus Annuus) Seedling. J. Plant Nutr. 2017, 40, 2326–2335. [Google Scholar] [CrossRef]
- Agnihotri, A.; Gupta, P.; Dwivedi, A.; Seth, C.S. Counteractive Mechanism (s) of Salicylic Acid in Response to Lead Toxicity in Brassica juncea (L.) Czern. Cv. Varuna. Planta 2018, 248, 49–68. [Google Scholar] [CrossRef]
- Zaid, A.; Mohammad, F.; Wani, S.H.; Siddique, K.M.H. Salicylic Acid Enhances Nickel Stress Tolerance by Up-Regulating Antioxidant Defense and Glyoxalase Systems in Mustard Plants. Ecotoxicol. Environ. Saf. 2019, 180, 575–587. [Google Scholar] [CrossRef]
- El Dakak, R.A.; Hassan, I.A. The Alleviative Effects of Salicylic Acid on Physiological Indices and Defense Mechanisms of Maize (Zea mays L. Giza 2) Stressed with Cadmium. Environ. Process. 2020, 7, 873–884. [Google Scholar] [CrossRef]
- Gupta, S.; Seth, C.S. Salicylic Acid Alleviates Chromium (VI) Toxicity by Restricting Its Uptake, Improving Photosynthesis and Augmenting Antioxidant Defense in Solanum lycopersicum L. Physiol. Mol. Biol. Plants 2021, 27, 2651–2664. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Wang, Y.; Li, G.-Z.; Hao, L. Salicylic Acid-Altering Arabidopsis Plant Response to Cadmium Exposure: Underlying Mechanisms Affecting Antioxidation and Photosynthesis-Related Processes. Ecotoxicol. Environ. Saf. 2019, 169, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Wael, M.S.; Mostafa, M.R.; Taia, A.A.E.-M.; Saad, M.H.; Magdi, T.A. Alleviation of Cadmium Toxicity in Common Bean (Phaseolus vulgaris L.) Plants by the Exogenous Application of Salicylic Acid. J. Hortic. Sci. Biotechnol. 2015, 90, 83–91. [Google Scholar] [CrossRef]
- Khalil, R.; Haroun, S.; Bassyoini, F.; Nagah, A.; Yusuf, M. Salicylic Acid in Combination with Kinetin or Calcium Ameliorates Heavy Metal Stress in Phaseolus Vulgaris Plant. J. Agric. Food Res. 2021, 5, 100182. [Google Scholar] [CrossRef]
- Belkadhi, A.; De Haro, A.; Obregon, S.; Chaïbi, W.; Djebali, W. Positive Effects of Salicylic Acid Pretreatment on the Composition of Flax Plastidial Membrane Lipids under Cadmium Stress. Environ. Sci. Pollut. Res. 2015, 22, 1457–1467. [Google Scholar] [CrossRef]
- Safari, F.; Akramian, M.; Salehi-Arjmand, H.; Khadivi, A. Physiological and Molecular Mechanisms Underlying Salicylic Acid-Mitigated Mercury Toxicity in Lemon Balm (Melissa officinalis L.). Ecotoxicol. Environ. Saf. 2019, 183, 109542. [Google Scholar] [CrossRef]
- Moravcová, Š.; Tůma, J.; Dučaiová, Z.K.; Waligórski, P.; Kula, M.; Saja, D.; Słomka, A.; Bąba, W.; Libik-Konieczny, M. Influence of Salicylic Acid Pretreatment on Seeds Germination and Some Defence Mechanisms of Zea mays Plants under Copper Stress. Plant Physiol. Biochem. 2018, 122, 19–30. [Google Scholar] [CrossRef]
- Zanganeh, R.; Jamei, R.; Rahmani, F. Modulation of Growth and Oxidative Stress by Seed Priming with Salicylic Acid in Zea mays L. under Lead Stress. J. Plant Interact. 2019, 14, 369–375. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic Acid-Induced Nitric Oxide Enhances Arsenic Toxicity Tolerance in Maize Plants by Upregulating the Ascorbate-Glutathione Cycle and Glyoxalase System. J. Hazard. Mater. 2020, 399, 123020. [Google Scholar] [CrossRef]
- Naz, R.; Sarfraz, A.; Anwar, Z.; Yasmin, H.; Nosheen, A.; Keyani, R.; Roberts, T.H. Combined Ability of Salicylic Acid and Spermidine to Mitigate the Individual and Interactive Effects of Drought and Chromium Stress in Maize (Zea mays L.). Plant Physiol. Biochem. 2021, 159, 285–300. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Yang, S.; Chen, Y. Salicylic Acid Alleviates Cadmium-Induced Inhibition of Growth and Photosynthesis through Upregulating Antioxidant Defense System in Two Melon Cultivars (Cucumis melo L.). Protoplasma 2015, 252, 911–924. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fotopoulos, V.; Nahar, K.; Fujita, M. Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 978-1-119-46866-0. [Google Scholar]
- Xu, L.L.; Fan, Z.Y.; Dong, Y.J.; Kong, J.; Bai, X.Y. Effects of Exogenous Salicylic Acid and Nitric Oxide on Physiological Characteristics of Two Peanut Cultivars under Cadmium Stress. Biol. Plant. 2015, 59, 171–182. [Google Scholar] [CrossRef]
- Kaya, C. Salicylic Acid-Induced Hydrogen Sulphide Improves Lead Stress Tolerance in Pepper Plants by Upraising the Ascorbate-Glutathione Cycle. Physiol Plant 2021, 173, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, G.; Wang, Y.; Dan, Y.; Guan, C.; Ji, J. Foliar Application of Salicylic Acid Alleviate the Cadmium Toxicity by Modulation the Reactive Oxygen Species in Potato. Ecotoxicol. Environ. Saf. 2019, 172, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Bano, K.; Kumar, B.; Alyemeni, M.N.; Ahmad, P. Exogenously-Sourced Salicylic Acid Imparts Resilience towards Arsenic Stress by Modulating Photosynthesis, Antioxidant Potential and Arsenic Sequestration in Brassica napus Plants. Antioxidants 2022, 11, 2010. [Google Scholar] [CrossRef]
- Singh, A.P.; Dixit, G.; Kumar, A.; Mishra, S.; Kumar, N.; Dixit, S.; Singh, P.K.; Dwivedi, S.; Trivedi, P.K.; Pandey, V.; et al. A Protective Role for Nitric Oxide and Salicylic Acid for Arsenite Phytotoxicity in Rice (Oryza sativa L.). Plant Physiol. Biochem. 2017, 115, 163–173. [Google Scholar] [CrossRef]
- Yotsova, E.K.; Dobrikova, A.G.; Stefanov, M.A.; Kouzmanova, M.; Apostolova, E.L. Improvement of the Rice Photosynthetic Apparatus Defence under Cadmium Stress Modulated by Salicylic Acid Supply to Roots. Theor. Exp. Plant Physiol. 2018, 30, 57–70. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ansary, M.M.U.; Fujita, M.; Tran, L.-S.P. Interactive Effects of Salicylic Acid and Nitric Oxide in Enhancing Rice Tolerance to Cadmium Stress. Int. J. Mol. Sci. 2019, 20, 5798. [Google Scholar] [CrossRef]
- Bai, X.; Dong, Y.; Kong, J.; Xu, L.; Liu, S. Effects of Application of Salicylic Acid Alleviates Cadmium Toxicity in Perennial Ryegrass. Plant Growth Regul. 2015, 75, 695–706. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, R.; Ren, X.; Jia, H.; Hua, L.; Xu, H.; Lv, X.; Zhao, J.; Wei, T. Effects of Salicylic Acid, Epi-Brassinolide and Calcium on Stress Alleviation and Cd Accumulation in Tomato Plants. Ecotoxicol. Environ. Saf. 2018, 157, 491–496. [Google Scholar] [CrossRef]
- Kirbag Zengin, F. Effects of Exogenous Salicylic Acid on Growth Characteristics and Biochemical Content of Wheat Seeds under Arsenic Stress. J. Environ. Biol. 2015, 36, 249–254. [Google Scholar]
- Nazar, R.; Iqbal, N.; Umar, S. Heat Stress Tolerance in Plants: Action of Salicylic Acid. In Salicylic Acid: A Multifaceted Hormone; Nazar, R., Iqbal, N., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 145–161. ISBN 978-981-10-6068-7. [Google Scholar]
- Iqbal, N.; Fatma, M.; Khan, N.A.; Umar, S. Chapter 28—Regulatory Role of Proline in Heat Stress Tolerance: Modulation by Salicylic Acid. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 437–448. ISBN 978-0-12-816451-8. [Google Scholar]
- Rai, K.K.; Pandey, N.; Rai, S.P. Salicylic Acid and Nitric Oxide Signaling in Plant Heat Stress. Physiol. Plant. 2020, 168, 241–255. [Google Scholar] [CrossRef]
- Sangwan, S.; Shameem, N.; Yashveer, S.; Tanwar, H.; Parray, J.A.; Jatav, H.S.; Sharma, S.; Punia, H.; Sayyed, R.Z.; Almalki, W.H.; et al. Role of Salicylic Acid in Combating Heat Stress in Plants: Insights into Modulation of Vital Processes. Front. Biosci.-Landmark 2022, 27, 310. [Google Scholar] [CrossRef]
- Janaagal, M.; Sharma, P.; Kumari, G.; Gulia, H.; Suresh, G.; Tallapragada, S.; Devi, S.; Lakra, N.; Arya, S.S.; Pooja, P. Revolutionizing High Temperature Stress Relief: Exploring the Latest Advances in Salicylic Acid Application. J. Crop Health 2024, 76, 1293–1305. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, K.; Gupta, A.K. Salicylic Acid Induces Differential Antioxidant Response in Spring Maize under High Temperature Stress. Indian J. Exp. Biol. 2016, 54, 386–393. [Google Scholar]
- Cingoz, G.S.; Gurel, E. Effects of Salicylic Acid on Thermotolerance and Cardenolide Accumulation under High Temperature Stress in Digitalis trojana Ivanina. Plant Physiol. Biochem. 2016, 105, 145–149. [Google Scholar] [CrossRef]
- Martel, A.B.; Qaderi, M.M. Does Salicylic Acid Mitigate the Adverse Effects of Temperature and Ultraviolet-B Radiation on Pea (Pisum sativum) Plants? Environ. Exp. Bot. 2016, 122, 39–48. [Google Scholar] [CrossRef]
- Zhang, C.X.; Feng, B.H.; Chen, T.T.; Zhang, X.F.; Tao, L.X.; Fu, G.F. Sugars, Antioxidant Enzymes and IAA Mediate Salicylic Acid to Prevent Rice Spikelet Degeneration Caused by Heat Stress. Plant Growth Regul. 2017, 83, 313–323. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, C.; Chen, T.; Zhang, X.; Tao, L.; Fu, G. Salicylic Acid Reverses Pollen Abortion of Rice Caused by Heat Stress. BMC Plant Biol. 2018, 18, 245. [Google Scholar] [CrossRef]
- Afzal, I.; Akram, M.W.; Rehman, H.U.; Rashid, S.; Basra, S.M.A. Moringa Leaf and Sorghum Water Extracts and Salicylic Acid to Alleviate Impacts of Heat Stress in Wheat. S. Afr. J. Bot. 2020, 129, 169–174. [Google Scholar] [CrossRef]
- Shah Jahan, M.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Exogenous Salicylic Acid Increases the Heat Tolerance in Tomato (Solanum lycopersicum L.) by Enhancing Photosynthesis Efficiency and Improving Antioxidant Defense System through Scavenging of Reactive Oxygen Species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- Zhang, Z.; Lan, M.; Han, X.; Wu, J.; Wang-Pruski, G. Response of Ornamental Pepper to High-Temperature Stress and Role of Exogenous Salicylic Acid in Mitigating High Temperature. J. Plant Growth Regul. 2020, 39, 133–146. [Google Scholar] [CrossRef]
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Cao, L.; Chen, L. Exogenous Salicylic Acid Ameliorates Heat Stress-Induced Damages and Improves Growth and Photosynthetic Efficiency in Alfalfa (Medicago sativa L.). Ecotoxicol. Environ. Saf. 2020, 191, 110206. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Janda, T. Multifaceted Role of Salicylic Acid in Combating Cold Stress in Plants: A Review. J. Plant Growth Regul. 2021, 40, 464–485. [Google Scholar] [CrossRef]
- Orabi, S.A.; Dawood, M.G.; Saleem, S.R. Comparative Study between the Physiological Role of Hydrogen Peroxide and Salicylic Acid in Alleviating Low Temperature Stress on Tomato Plants Grown under Sandponic Culture. Sci. Agric. 2015, 9, 49–59. [Google Scholar] [CrossRef]
- Mutlu, S.; Atıcı, Ö.; Nalbantoğlu, B.; Mete, E. Exogenous Salicylic Acid Alleviates Cold Damage by Regulating Antioxidative System in Two Barley (Hordeum vulgare L.) Cultivars. Front. Life Sci. 2016, 9, 99–109. [Google Scholar] [CrossRef]
- Huang, C.; Wang, D.; Sun, L.; Wei, L. Effects of Exogenous Salicylic Acid on the Physiological Characteristics of Dendrobium Officinale under Chilling Stress. Plant Growth Regul. 2016, 79, 199–208. [Google Scholar] [CrossRef]
- Keshavarz, H.; Modarres Sanavy, S.A.M.; Sadegh Ghol Moghadam, R. Impact of Foliar Application with Salicylic Acid on Biochemical Characters of Canola Plants under Cold Stress Condition. Not. Sci. Biol. 2016, 8, 98–105. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Alleviation of Field Low-Temperature Stress in Winter Wheat by Exogenous Application of Salicylic Acid. J. Plant Growth Regul. 2021, 40, 811–823. [Google Scholar] [CrossRef]
- Wang, X.; Miao, J.; Kang, W.; Shi, S. Exogenous Application of Salicylic Acid Improves Freezing Stress Tolerance in Alfalfa. Front. Plant Sci. 2023, 14, 1091077. [Google Scholar] [CrossRef]
- Per, T.S.; Fatma, M.; Asgher, M.; Javied, S.; Khan, N.A. Salicylic Acid and Nutrients Interplay in Abiotic Stress Tolerance. In Salicylic Acid: A Multifaceted Hormone; Nazar, R., Iqbal, N., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 221–237. ISBN 978-981-10-6068-7. [Google Scholar]
- Namdjoyan, S.; Kermanian, H.; Abolhasani Soorki, A.; Modarres Tabatabaei, S.; Elyasi, N. Interactive Effects of Salicylic Acid and Nitric Oxide in Alleviating Zinc Toxicity of Safflower (Carthamus tinctorius L.). Ecotoxicology 2017, 26, 752–761. [Google Scholar] [CrossRef]
- Metwally, A.M.; Radi, A.A.; El-Shazoly, R.M.; Hamada, A.M. The Role of Calcium, Silicon and Salicylic Acid Treatment in Protection of Canola Plants against Boron Toxicity Stress. J. Plant Res. 2018, 131, 1015–1028. [Google Scholar] [CrossRef]
- El-Shazoly, R.M.; Metwally, A.A.; Hamada, A.M. Salicylic Acid or Thiamin Increases Tolerance to Boron Toxicity Stress in Wheat. J. Plant Nutr. 2019, 42, 702–722. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mohamed, H.I.; Mahmoud, A.; Elkelish, A.; Misra, A.N.; Guy, K.M.; Kamran, M.; Ai, S.; Zhang, M. Salicylic Acid Stimulates Antioxidant Defense and Osmolyte Metabolism to Alleviate Oxidative Stress in Watermelons under Excess Boron. Plants 2020, 9, 724. [Google Scholar] [CrossRef]
- Farghaly, F.A.; Salam, H.K.; Hamada, A.M.; Radi, A.A. The Role of Benzoic Acid, Gallic Acid and Salicylic Acid in Protecting Tomato Callus Cells from Excessive Boron Stress. Sci. Hortic. 2021, 278, 109867. [Google Scholar] [CrossRef]
- Deus, A.C.F.; de Mello Prado, R.; de Cássia Félix Alvarez, R.; de Oliveira, R.L.L.; Felisberto, G. Role of Silicon and Salicylic Acid in the Mitigation of Nitrogen Deficiency Stress in Rice Plants. Silicon 2020, 12, 997–1005. [Google Scholar] [CrossRef]
- Li, Z.; Fan, R.; Peng, X.; Shu, J.; Liu, L.; Wang, J.; Lin, L. Salicylic Acid Alleviates Selenium Stress and Promotes Selenium Uptake of Grapevine. Physiol. Mol. Biol. Plants 2022, 28, 625–635. [Google Scholar] [CrossRef]
- Nie, W.; Gong, B.; Chen, Y.; Wang, J.; Wei, M.; Shi, Q. Photosynthetic Capacity, Ion Homeostasis and Reactive Oxygen Metabolism Were Involved in Exogenous Salicylic Acid Increasing Cucumber Seedlings Tolerance to Alkaline Stress. Sci. Hortic. 2018, 235, 413–423. [Google Scholar] [CrossRef]
- Khan, A.; Kamran, M.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Al-Amri, I.; Lee, I.-J.; Khan, A.L. Silicon and Salicylic Acid Confer High-pH Stress Tolerance in Tomato Seedlings. Sci. Rep. 2019, 9, 19788. [Google Scholar] [CrossRef]
- Schenk, S.T.; Schikora, A. AHL-Priming Functions via Oxylipin and Salicylic Acid. Front. Plant Sci. 2015, 5, 784. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Mohamed, H.I.; El-Shazly, H.H.; Badr, A. Role of Salicylic Acid in Biotic and Abiotic Stress Tolerance in Plants. In Plant Phenolics in Sustainable Agriculture: Volume 1; Lone, R., Shuab, R., Kamili, A.N., Eds.; Springer: Singapore, 2020; pp. 533–554. ISBN 9789811548901. [Google Scholar]
- Maruri-López, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and Extracellular Journey of the Phytohormone Salicylic Acid. Front. Plant Sci. 2019, 10, 423. [Google Scholar] [CrossRef]
- Urban, L.; Lauri, F.; Ben Hdech, D.; Aarrouf, J. Prospects for Increasing the Efficacy of Plant Resistance Inducers Stimulating Salicylic Acid. Agronomy 2022, 12, 3151. [Google Scholar] [CrossRef]
- Herrera-Vásquez, A.; Salinas, P.; Holuigue, L. Salicylic Acid and Reactive Oxygen Species Interplay in the Transcriptional Control of Defense Genes Expression. Front. Plant Sci. 2015, 6, 171. [Google Scholar] [CrossRef]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic Acid in Relation to Other Phytohormones in Plant: A Study towards Physiology and Signal Transduction under Challenging Environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Chen, Y.E.; Cui, J.M.; Li, G.X.; Yuan, M.; Zhang, Z.W.; Yuan, S.; Zhang, H.Y. Effect of Salicylic Acid on the Antioxidant System and Photosystem II in Wheat Seedlings. Biol. Plant. 2016, 60, 139–147. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Emam, Y. Modulation of Oxidative Damage Due to Salt Stress Using Salicylic Acid in Hordeum vulgare. Arch. Agron. Soil Sci. 2018, 64, 1268–1277. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Influence of Exogenous Salicylic Acid and Nitric Oxide on Growth, Photosynthesis, and Ascorbate-Glutathione Cycle in Salt Stressed Vigna Angularis. Biomolecules 2020, 10, 42. [Google Scholar] [CrossRef]
- Lukan, T.; Coll, A. Intertwined Roles of Reactive Oxygen Species and Salicylic Acid Signaling Are Crucial for the Plant Response to Biotic Stress. Int. J. Mol. Sci. 2022, 23, 5568. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Corpas, F.J.; Ahmad, P. Nitric Oxide, Salicylic Acid and Oxidative Stress: Is It a Perfect Equilateral Triangle? Plant Physiol. Biochem. 2022, 184, 56–64. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.-D.S.; Moustaka, J.; İşgören, S.; Şaş, B. Harnessing the Role of Foliar Applied Salicylic Acid in Decreasing Chlorophyll Content to Reassess Photosystem II Photoprotection in Crop Plants. Int. J. Mol. Sci. 2022, 23, 7038. [Google Scholar] [CrossRef]
- Myers, R.J., Jr.; Fichman, Y.; Zandalinas, S.I.; Mittler, R. Jasmonic Acid and Salicylic Acid Modulate Systemic Reactive Oxygen Species Signaling during Stress Responses. Plant Physiol. 2023, 191, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Spormann, S.; Soares, C.; Fidalgo, F. Salicylic Acid Alleviates Glyphosate-Induced Oxidative Stress in Hordeum vulgare L. J. Environ. Manag. 2019, 241, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Radwan, D.E.M.; Mohamed, A.K.; Fayez, K.A.; Abdelrahman, A.M. Oxidative Stress Caused by Basagran® Herbicide Is Altered by Salicylic Acid Treatments in Peanut Plants. Heliyon 2019, 5, e01791. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Wang, C.; Li, Q.; Ji, J.; Wang, G.; Jin, C.; Tong, Y. LcSABP2, a Salicylic Acid Binding Protein 2 Gene from Lycium Chinense, Confers Resistance to Triclosan Stress in Nicotiana tabacum. Ecotoxicol. Environ. Saf. 2019, 183, 109516. [Google Scholar] [CrossRef]
- Liu, T.; Li, T.; Zhang, L.; Li, H.; Liu, S.; Yang, S.; An, Q.; Pan, C.; Zou, N. Exogenous Salicylic Acid Alleviates the Accumulation of Pesticides and Mitigates Pesticide-Induced Oxidative Stress in Cucumber Plants (Cucumis sativus L.). Ecotoxicol. Environ. Saf. 2021, 208, 111654. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, P.K.; Singh, S.; Singh, A. An Overview on the Modulation of Pesticide Detoxification Mechanism via Salicylic Acid in the Plants. Environ. Pollut. Bioavailab. 2023, 35, 2242701. [Google Scholar] [CrossRef]
- Gorni, P.H.; Pacheco, A.C. Growth Promotion and Elicitor Activity of Salicylic Acid in Achillea millefolium L. Afr. J. Biotechnol. 2016, 15, 657–665. [Google Scholar] [CrossRef]
- Su, H.; Song, S.; Yan, X.; Fang, L.; Zeng, B.; Zhu, Y. Endogenous Salicylic Acid Shows Different Correlation with Baicalin and Baicalein in the Medicinal Plant Scutellaria Baicalensis Georgi Subjected to Stress and Exogenous Salicylic Acid. PLoS ONE 2018, 13, e0192114. [Google Scholar] [CrossRef]
- Ali, B. Salicylic Acid: An Efficient Elicitor of Secondary Metabolite Production in Plants. Biocatal. Agric. Biotechnol. 2021, 31, 101884. [Google Scholar] [CrossRef]
- Shatpathy, P.; Kar, M.; Dwibedi, S.K.; Dash, A. Seed Priming with Salicylic Acid Improves Germination and Seedling Growth of Rice (Oryza sativa L.) under PEG-6000 Induced Water Stress. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 907–924. [Google Scholar] [CrossRef]
- Mahmood-ur-Rehman, M.; Amjad, M.; Ziaf, K.; Ahmad, R. Seed Priming with Salicylic Acid Improve Seed Germination and Physiological Responses of Carrot Seeds. Pak. J. Agric. Sci. 2020, 57, 351–359. [Google Scholar]
- Galviz-Fajardo, Y.C.; Bortolin, G.S.; Deuner, S.; Amarante, L.D.; Reolon, F.; Moraes, D.M.D. Seed Priming with Salicylic Acid Potentiates Water Restriction-Induced Effects in Tomato Seed Germination and Early Seedling Growth. J. Seed Sci. 2020, 42, e202042031. [Google Scholar] [CrossRef]
- Ceritoğlu, M.; Erman, M. Mitigation of Salinity Stress on Chickpea Germination by Salicylic Acid Priming. Uluslararası Tarım Ve Yaban Hayatı Bilim. Derg. 2020, 6, 582–591. [Google Scholar] [CrossRef]
- Szalai, G.; Pál, M.; Árendás, T.; Janda, T. Priming Seed with Salicylic Acid Increases Grain Yield and Modifies Polyamine Levels in Maize. Cereal Res. Commun. 2016, 44, 537–548. [Google Scholar] [CrossRef]
- Mahesh, H.M.; Murali, M.; Anup Chandra Pal, M.; Melvin, P.; Sharada, M.S. Salicylic Acid Seed Priming Instigates Defense Mechanism by Inducing PR-Proteins in Solanum melongena L. upon Infection with Verticillium dahliae Kleb. Plant Physiol. Biochem. 2017, 117, 12–23. [Google Scholar] [CrossRef]
- Zanganeh, R.; Jamei, R.; Rahmani, F. Impacts of Seed Priming with Salicylic Acid and Sodium Hydrosulfide on Possible Metabolic Pathway of Two Amino Acids in Maize Plant under Lead Stress. Mol. Biol. Res. Commun. 2018, 7, 83–88. [Google Scholar] [CrossRef]
- Bortolin, G.S.; Teixeira, S.B.; de Mesquita Pinheiro, R.; Ávila, G.E.; Carlos, F.S.; da Silva Pedroso, C.E.; Deuner, S. Seed Priming with Salicylic Acid Minimizes Oxidative Effects of Aluminum on Trifolium Seedlings. J. Soil Sci. Plant Nutr. 2020, 20, 2502–2511. [Google Scholar] [CrossRef]
- Methenni, K.; Abdallah, M.B.; Nouairi, I.; Smaoui, A.; Zarrouk, M.; Youssef, N.B. Salicylic Acid and Calcium Pretreatments Alleviate the Toxic Effect of Salinity in the Oueslati Olive Variety. Sci. Hortic. 2018, 233, 349–358. [Google Scholar] [CrossRef]
- Gharbi, E.; Lutts, S.; Dailly, H.; Quinet, M. Comparison between the Impacts of Two Different Modes of Salicylic Acid Application on Tomato (Solanum lycopersicum) Responses to Salinity. Plant Signal. Behav. 2018, 13, e1469361. [Google Scholar] [CrossRef]
- Ghafoor, M.F.; Ali, Q.; Malik, A. Effects of Salicylic Acid Priming for Salt Stress Tolerance in Wheat. Biol. Clin. Sci. Res. J. 2020, 2020, e024. [Google Scholar] [CrossRef]
- Iqbal, S.; Ali, Q.; Malik, A. Effects of Seed Priming with Salicylic Acid on Zea Mays Seedlings Grown Under Salt Stress Conditions. Biol. Clin. Sci. Res. J. 2021, 65. [Google Scholar] [CrossRef]
- Islam, A.T.M.T.; Ullah, H.; Himanshu, S.K.; Tisarum, R.; Cha-um, S.; Datta, A. Effect of Salicylic Acid Seed Priming on Morpho-Physiological Responses and Yield of Baby Corn under Salt Stress. Sci. Hortic. 2022, 304, 111304. [Google Scholar] [CrossRef]
- Ahmad, F.; Kamal, A.; Singh, A.; Ashfaque, F.; Alamri, S.; Siddiqui, M.H. Salicylic Acid Modulates Antioxidant System, Defense Metabolites, and Expression of Salt Transporter Genes in Pisum Sativum Under Salinity Stress. J. Plant Growth Regul. 2022, 41, 1905–1918. [Google Scholar] [CrossRef]
- Ben Youssef, R.; Boukari, N.; Abdelly, C.; Jelali, N. Mitigation of Salt Stress and Stimulation of Growth by Salicylic Acid and Calcium Chloride Seed Priming in Two Barley Species. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2023, 157, 758–768. [Google Scholar] [CrossRef]
- Maqsood, M.F.; Shahbaz, M.; Zulfiqar, U.; Saman, R.U.; Rehman, A.; Naz, N.; Akram, M.; Haider, F.U. Enhancing Wheat Growth and Yield through Salicylic Acid-Mediated Regulation of Gas Exchange, Antioxidant Defense, and Osmoprotection under Salt Stress. Stresses 2023, 3, 372–386. [Google Scholar] [CrossRef]
- Ayub, Q.; Khan, S.M.; Mehmood, A.; Haq, N.U.; Ali, S.; Ahmad, T.; Shoukat, M.F. Enhancement of Physiological and Biochemical Attributes of Okra by Application of Salicylic Acid under Drought Stress. J. Hortic. Sci. Technol. 2020, 3, 113–119. [Google Scholar] [CrossRef]
- Tayyab, N.; Naz, R.; Yasmin, H.; Nosheen, A.; Keyani, R.; Sajjad, M.; Hassan, M.N.; Roberts, T.H. Combined Seed and Foliar Pre-Treatments with Exogenous Methyl Jasmonate and Salicylic Acid Mitigate Drought-Induced Stress in Maize. PLoS ONE 2020, 15, e0232269. [Google Scholar] [CrossRef]
- Alam, M.U.; Fujita, M.; Nahar, K.; Rahman, A.; Anee, T.I.; Masud, A.A.C.; Amin, A.K.M.R.; Hasanuzzaman, M. Seed Priming Upregulates Antioxidant Defense and Glyoxalase Systems to Conferring Simulated Drought Tolerance in Wheat Seedlings. Plant Stress 2022, 6, 100120. [Google Scholar] [CrossRef]
- Kulak, M.; Jorrín-Novo, J.V.; Romero-Rodriguez, M.C.; Yildirim, E.D.; Gul, F.; Karaman, S. Seed Priming with Salicylic Acid on Plant Growth and Essential Oil Composition in Basil (Ocimum basilicum L.) Plants Grown under Water Stress Conditions. Ind. Crops Prod. 2021, 161, 113235. [Google Scholar] [CrossRef]
- Chakma, R.; Biswas, A.; Saekong, P.; Ullah, H.; Datta, A. Foliar Application and Seed Priming of Salicylic Acid Affect Growth, Fruit Yield, and Quality of Grape Tomato under Drought Stress. Sci. Hortic. 2021, 280, 109904. [Google Scholar] [CrossRef]
- Ahmed, M.; Tóth, Z.; Decsi, K. The Impact of Salinity on Crop Yields and the Confrontational Behavior of Transcriptional Regulators, Nanoparticles, and Antioxidant Defensive Mechanisms under Stressful Conditions: A Review. Int. J. Mol. Sci. 2024, 25, 2654. [Google Scholar] [CrossRef] [PubMed]
- Souri, Z.; Karimi, N.; Sarmadi, M.; Rostami, E. Salicylic Acid Nanoparticles (SANPs) Improve Growth and Phytoremediation Efficiency of Isatis cappadocica Desv., under As Stress. IET Nanobiotechnol. 2017, 11, 650–655. [Google Scholar] [CrossRef]
- Mozafari, A.A.; Havas, F.; Ghaderi, N. Application of Iron Nanoparticles and Salicylic Acid in in Vitro Culture of Strawberries (Fragaria × ananassa Duch.) to Cope with Drought Stress. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 132, 511–523. [Google Scholar] [CrossRef]
- Abdoli, S.; Ghassemi-Golezani, K.; Alizadeh-Salteh, S. Responses of Ajowan (Trachyspermum ammi L.) to Exogenous Salicylic Acid and Iron Oxide Nanoparticles under Salt Stress. Environ. Sci. Pollut. Res. 2020, 27, 36939–36953. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Abdoli, S. Improving ATPase and PPase Activities, Nutrient Uptake and Growth of Salt Stressed Ajowan Plants by Salicylic Acid and Iron-Oxide Nanoparticles. Plant Cell Rep. 2021, 40, 559–573. [Google Scholar] [CrossRef]
- Faizan, M.; Sehar, S.; Rajput, V.D.; Faraz, A.; Afzal, S.; Minkina, T.; Sushkova, S.; Adil, M.F.; Yu, F.; Alatar, A.A.; et al. Modulation of Cellular Redox Status and Antioxidant Defense System after Synergistic Application of Zinc Oxide Nanoparticles and Salicylic Acid in Rice (Oryza sativa) Plant under Arsenic Stress. Plants 2021, 10, 2254. [Google Scholar] [CrossRef]
- Al-Taey, D.K.A.; Al-Musawi, Z.J.M. The Impact of Nano Fertilization and Salicylic Acid on Growth, Yield and Anti-Oxidant Contents in Rocket Plant under Salt Stress. Agraarteadus 2022, 33, 43–47. [Google Scholar] [CrossRef]
- Zhong, X.; Su, G.; Zeng, Q.; Li, G.; Xu, H.; Wu, H.; Zhou, H.; Zhou, X. Preparation of Salicylic Acid-Functionalized Nanopesticides and Their Applications in Enhancing Salt Stress Resistance. ACS Appl. Mater. Interfaces 2023, 15, 43282–43293. [Google Scholar] [CrossRef]
- Aazami, M.A.; Maleki, M.; Rasouli, F.; Gohari, G. Protective Effects of Chitosan Based Salicylic Acid Nanocomposite (CS-SA NCs) in Grape (Vitis vinifera Cv. ‘Sultana’) under Salinity Stress. Sci. Rep. 2023, 13, 883. [Google Scholar] [CrossRef]
- Yin, J.; Zhao, J.; Wang, Z.; Xue, F.; Wang, Q.; Guo, H.; Cheng, H.; Li, J.; Shen, J.; Yin, M.; et al. Preparation of Salicylic Acid Nano-Protectant with Dual Synergistic Mechanism: High Direct Fungicidal Activity and Plant Defence toward Cotton Verticillium Wilt. Chem. Eng. J. 2024, 496, 154036. [Google Scholar] [CrossRef]
- Sedaghat, M.; Tahmasebi-Sarvestani, Z.; Emam, Y.; Mokhtassi-Bidgoli, A. Physiological and Antioxidant Responses of Winter Wheat Cultivars to Strigolactone and Salicylic Acid in Drought. Plant Physiol. Biochem. 2017, 119, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, M.; Sarvestani, Z.T.; Emam, Y.; Bidgoli, A.M.; Sorooshzadeh, A. Foliar-Applied GR24 and Salicylic Acid Enhanced Wheat Drought Tolerance. Russ. J. Plant Physiol. 2020, 67, 733–739. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Niazi, A.; Pessarakli, M.; Arvin, M.J. P5CS Expression Level and Proline Accumulation in the Sensitive and Tolerant Wheat Cultivars under Control and Drought Stress Conditions in the Presence/Absence of Silicon and Salicylic Acid. J. Plant Interact. 2018, 13, 461–471. [Google Scholar] [CrossRef]
- Khalequzzaman; Ullah, H.; Himanshu, S.K.; García-Caparrós, P.; Praseartkul, P.; Tisarum, R.; Cha-um, S.; Datta, A. Exogenous Silicon and Salicylic Acid Applications Enhance Growth, Yield, and Physiological Traits of Cotton Plants under Drought Stress. J. Soil Sci. Plant Nutr. 2024, 24, 5947–5960. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Interaction of 24-Epibrassinolide and Salicylic Acid Regulates Pigment Contents, Antioxidative Defense Responses, and Gene Expression in Brassica juncea L. Seedlings under Pb Stress. Environ. Sci. Pollut. Res. 2018, 25, 15159–15173. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Arnao, M.B. Relationship of Melatonin and Salicylic Acid in Biotic/Abiotic Plant Stress Responses. Agronomy 2018, 8, 33. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Moosa, A.; Ferrante, A.; Nafees, M.; Darras, A.; Nazir, M.M.; AlShaqhaa, M.A.; Elsaid, F.G. Melatonin and Salicylic Acid Synergistically Improve Arsenic Induced Oxidative Stress Tolerance in Ornamental Sword Lily. Sci. Hortic. 2023, 322, 112389. [Google Scholar] [CrossRef]
- Rafique, N.; Ilyas, N.; Aqeel, M.; Raja, N.I.; Shabbir, G.; Ajaib, M.; Sayyed, R.Z.; Alharbi, S.A.; Ansari, M.J. Interactive Effects of Melatonin and Salicylic Acid on Brassica napus under Drought Condition. Plant Soil 2024, 505, 65–84. [Google Scholar] [CrossRef]
- Bijanzadeh, E.; Naderi, R.; Egan, T.P. Exogenous Application of Humic Acid and Salicylic Acid to Alleviate Seedling Drought Stress in Two Corn (Zea mays L.) Hybrids. J. Plant Nutr. 2019, 42, 1483–1495. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Sami, F.; Siddiqui, H.; Hayat, S. Supplementation of Salicylic Acid and Citric Acid for Alleviation of Cadmium Toxicity to Brassica juncea. J. Plant Growth Regul. 2020, 39, 641–655. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F. The Role of Salicylic Acid and Gibberellin Signaling in Plant Responses to Abiotic Stress with an Emphasis on Heavy Metals. Plant Signal. Behav. 2020, 15, 1777372. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Tsuda, K. Salicylic acid and jasmonic acid crosstalk in plant immunity. Essays Biochem. 2022, 66, 647–656. [Google Scholar] [PubMed]
- Sofy, M.R.; Seleiman, M.F.; Alhammad, B.A.; Alharbi, B.M.; Mohamed, H.I. Minimizing Adverse Effects of Pb on Maize Plants by Combined Treatment with Jasmonic, Salicylic Acids and Proline. Agronomy 2020, 10, 699. [Google Scholar] [CrossRef]
- Prakash, V.; Singh, V.P.; Tripathi, D.K.; Sharma, S.; Corpas, F.J. Nitric Oxide (NO) and Salicylic Acid (SA): A Framework for Their Relationship in Plant Development under Abiotic Stress. Plant Biol. 2021, 23, 39–49. [Google Scholar] [CrossRef]
- Rai, K.K.; Pandey, N.; Rai, N.; Rai, S.K.; Pandey-Rai, S. Salicylic Acid and Nitric Oxide: Insight Into the Transcriptional Regulation of Their Metabolism and Regulatory Functions in Plants. Front. Agron. 2021, 3, 781027. [Google Scholar] [CrossRef]
- Rasheed, F.; Anjum, N.A.; Masood, A.; Sofo, A.; Khan, N.A. The Key Roles of Salicylic Acid and Sulfur in Plant Salinity Stress Tolerance. J. Plant Growth Regul. 2022, 41, 1891–1904. [Google Scholar] [CrossRef]
- Munsif, F.; Shah, T.; Arif, M.; Jehangir, M.; Afridi, M.Z.; Ahmad, I.; Jan, B.L.; Alansi, S. Combined Effect of Salicylic Acid and Potassium Mitigates Drought Stress through the Modulation of Physio-Biochemical Attributes and Key Antioxidants in Wheat. Saudi J. Biol. Sci. 2022, 29, 103294. [Google Scholar] [CrossRef]
- Mabudi Bilasvar, H.; Ghassemi-Golezani, K.; Mohammadi Nassab, A.D. Seed Development, Oil Accumulation and Fatty Acid Composition of Drought Stressed Rapeseed Plants Affected by Salicylic Acid and Putrescine. Gesunde Pflanz. 2022, 74, 333–345. [Google Scholar] [CrossRef]
- Benjamin, G.; Pandharikar, G.; Frendo, P. Salicylic Acid in Plant Symbioses: Beyond Plant Pathogen Interactions. Biology 2022, 11, 861. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Riaz, M.; Shahzad, S.M.; Imran, Q.; Ali, I. Combined Ability of Chromium (Cr) Tolerant Plant Growth Promoting Bacteria (PGPB) and Salicylic Acid (SA) in Attenuation of Chromium Stress in Maize Plants. Plant Physiol. Biochem. 2016, 108, 456–467. [Google Scholar] [CrossRef]
- Khan, N.; Zandi, P.; Ali, S.; Mehmood, A.; Adnan Shahid, M.; Yang, J. Impact of Salicylic Acid and PGPR on the Drought Tolerance and Phytoremediation Potential of Helianthus Annus. Front. Microbiol. 2018, 9, 2507. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Bharti, A. Salicylic Acid Improves Arbuscular Mycorrhizal Symbiosis, and Chickpea Growth and Yield by Modulating Carbohydrate Metabolism under Salt Stress. Mycorrhiza 2018, 28, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Azmat, A.; Yasmin, H.; Hassan, M.N.; Nosheen, A.; Naz, R.; Sajjad, M.; Ilyas, N.; Akhtar, M.N. Co-Application of Bio-Fertilizer and Salicylic Acid Improves Growth, Photosynthetic Pigments and Stress Tolerance in Wheat under Drought Stress. PeerJ 2020, 8, e9960. [Google Scholar] [CrossRef] [PubMed]
- Nigam, B.; Dubey, R.S.; Rathore, D. Protective Role of Exogenously Supplied Salicylic Acid and PGPB (Stenotrophomonas sp.) on Spinach and Soybean Cultivars Grown under Salt Stress. Sci. Hortic. 2022, 293, 110654. [Google Scholar] [CrossRef]
- Boamah, S.; Ojangba, T.; Zhang, S.; Zhu, N.; Osei, R.; John Tiika, R.; Boakye, T.A.; Khurshid, A.; Inayat, R.; Effah, Z.; et al. Evaluation of Salicylic Acid (SA) Signaling Pathways and Molecular Markers in Trichoderma-Treated Plants under Salinity and Fusarium Stresses. A Review. Eur. J. Plant Pathol. 2023, 166, 259–274. [Google Scholar] [CrossRef]
- Shaukat, K.; Zahra, N.; Hafeez, M.B.; Naseer, R.; Batool, A.; Batool, H.; Raza, A.; Wahid, A. Chapter 2—Role of Salicylic Acid–Induced Abiotic Stress Tolerance and Underlying Mechanisms in Plants. In Emerging Plant Growth Regulators in Agriculture; Aftab, T., Naeem, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 73–98. ISBN 978-0-323-91005-7. [Google Scholar]
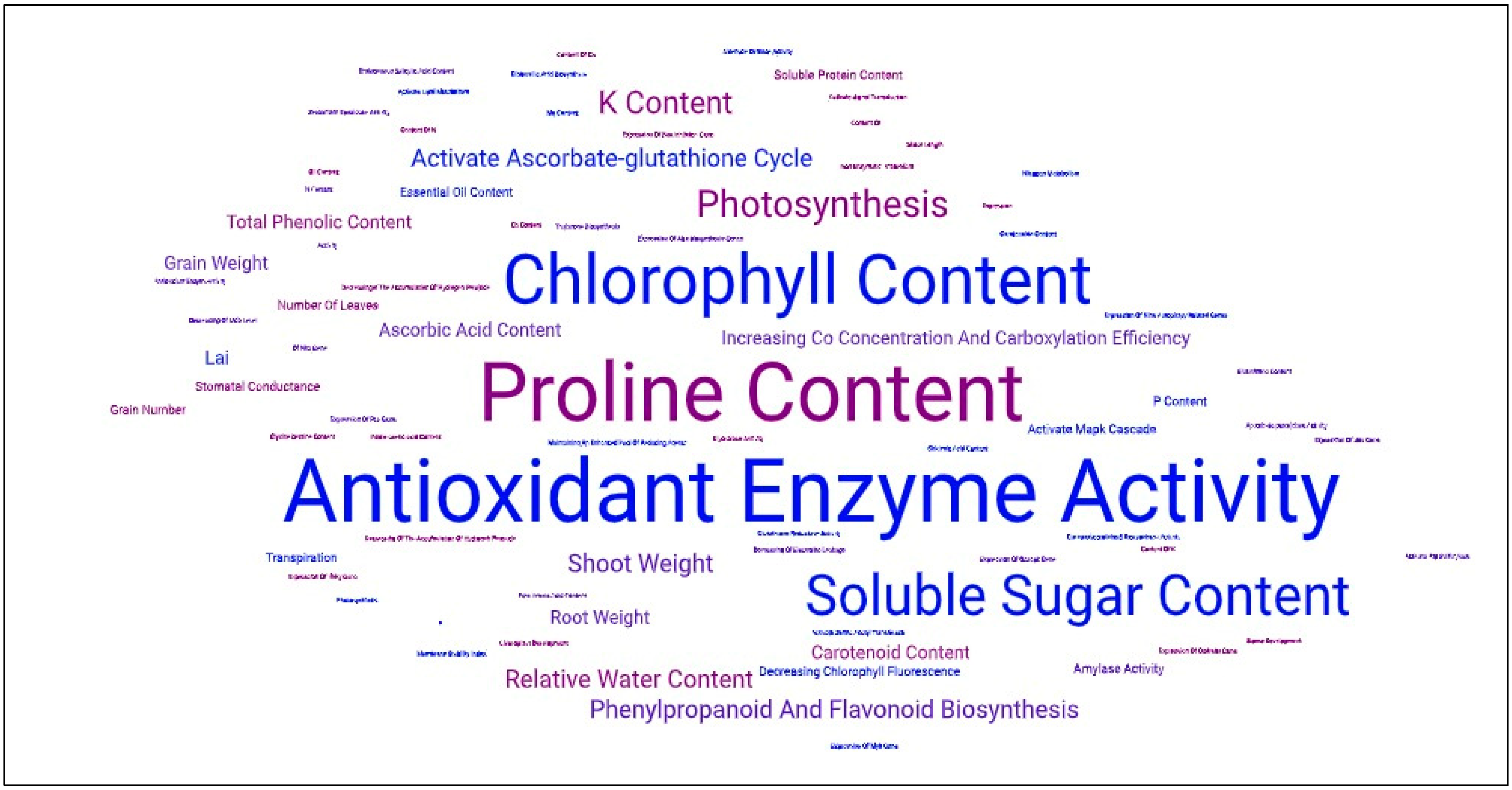
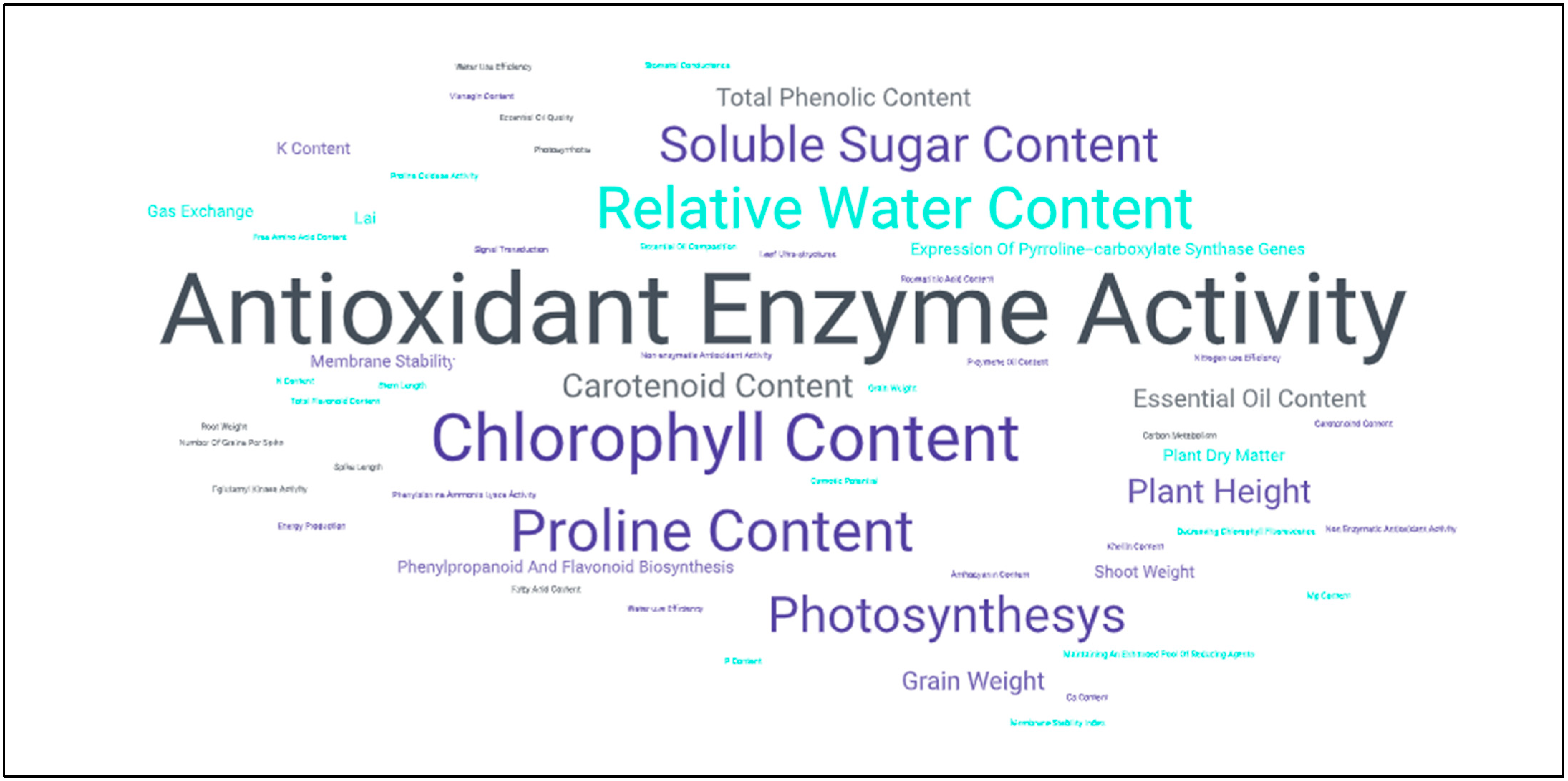
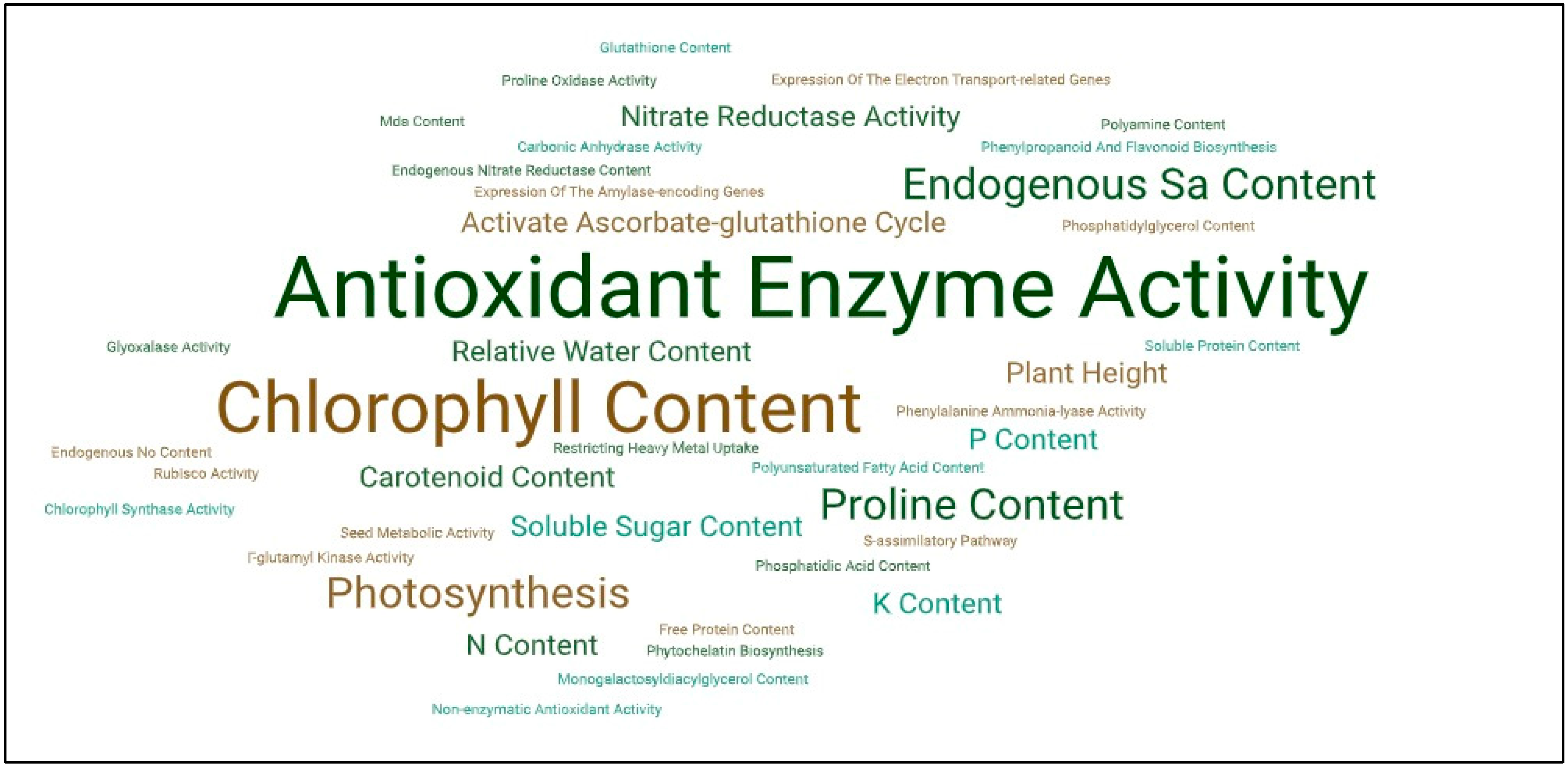
| Plant Species | Target Areas of Beneficial Effect | References |
|---|---|---|
| Barley | Grain number | [64] |
| Proline content, indole-acetic acid content | [65] | |
| Barley and Wheat | Higher grain yield | [64] |
| Bean | Concentrations of total chlorophylls, total carotenoids, total soluble sugars, free proline and ascorbic acid, contents of N, P, K, and Ca, and ratios of K/Na and Ca/Na | [66] |
| Improve shoot length, number, and area of leaves per plant, plant dry weight | [67] | |
| Free proline, soluble sugars, chlorophyll fluorescence, relative water content, and membrane stability index | ||
| Maintaining an enhanced pool of reducing agents | [68] | |
| Leaf pigment contents, ascorbic acid, glutathione, proline, K, and antioxidant enzyme contents | [69] | |
| Canola | Shoot fresh weight, root dry weight, chlorophyll content, and antioxidant enzyme activities | [70] |
| Chinese Cabbage | Photosynthetic performance, productivity, and photosynthetic pigment content | [71] |
| Cotton | Increasing K+ and Mg2+ absorption and osmotic regulators accumulation | [72] |
| Photosynthetic parameters, yield parameters | [69] | |
| Decrease in the accumulation of hydrogen peroxide, and the elevated levels of electrolyte leakage and malondialdehyde | [73] | |
| Cowpeas | Total free amino acids and shikimic acid | [74] |
| Cucumber | Regulate endogenous salicylic acid levels | [75] |
| Damask Rose | Activity of enzymatic and non-enzymatic defense systems | [76] |
| Dianthus superbus | Stoma and chloroplast development | [77] |
| Leaf biomass, soluble protein, sugar content, expression of MYB and P5CS genes | ||
| Eggplant | Activate pigments | [78] |
| Egletes viscosa | Increase in the content of all organic compounds, decrease in H2O2 overproduction | [79] |
| Ethiopian Mustard | Proline content, antioxidant enzyme activities | [80] |
| Fenugreek Plants | Decrease of calcium, potassium, and phosphorus concentrations | [81] |
| Feverfew | Increase in the essential oil, sugar, and antioxidant contents | [82] |
| Limonium bicolor | High levels of gibberellic acid (GA) and high levels of amylase and α-amylase activity | [83] |
| Maize | Proline concentration, amino acid accumulation | [84] |
| Root and shoot growth, increased CO2 concentration | [85] | |
| Regulation of phytochromes and various organic and inorganic osmolytes | [86] | |
| Leaf osmolyte and sugar contents | [87] | |
| Activate the ascorbate–glutathione cycle | [88] | |
| Melon | Activate MAPK cascade, plant hormone signal transduction, lipid metabolism, biosynthesis of secondary metabolites (phenylpropanoids and flavonoids) | [89] |
| Mentha Pulegium | Relative water content | [90] |
| Mungbean | Increasing photosystem II activity, decreasing fluorescence | [91] |
| Photosynthetic efficiency | [92] | |
| Mustard | Increase in ATP sulfurylase and serine acetyl transferase activity | [93] |
| Neutralize the NaCl stress-induced suppression | [94] | |
| Nitraria tangutorum | Antioxidant capacity, ascorbate–glutathione cycle | [95] |
| Okra | Antioxidant enzyme activity | [96] |
| Improving plant properties in hydroponic system | [97] | |
| Olive | Chlorophyll index | [98] |
| Pea | Superoxide dismutase, catalase, guaiacol peroxidase and ascorbate peroxidase activity | [99] |
| Pearl Millet and Wheat | Increase of the dissipation flux in the photosynthesis | [100] |
| Pennyroyal | Content and antioxidant activity of essential oils, nutrient uptake, photosynthetic activity, plant growth | [101] |
| Pepper | Upregulate the activities of ascorbate–glutathione cycle | [102] |
| Pomegranate | Chlorophyll, total phenolic, carbohydrate, and proline content | [103] |
| Radish | Accumulation of compatible solutes, antioxidant activities | [104] |
| Rice | Expression of SOS1 and NHX1 genes | [105] |
| MAPK-1, transcription factor WRKY53, Bax Inhibitor-1, and nine Autophagy Related Genes were upregulated | [106] | |
| Regulation of the expression of osdreb2a and ossapk8 genes | [107] | |
| Rosemary | Total phenolic, chlorophyll, carbohydrates, and proline contents of leaves | [108] |
| Safflower | Relative water content, leaf area index, and chlorophyll content index | [109] |
| Content of total phenolics, total flavonoids, total flavonols, anthocyanins, and antioxidant activity | [110] | |
| Increasing glycine betaine and anthocyanin content | [111] | |
| Chlorophyll content, antioxidant capacity | [112] | |
| Sage | Appearance of the new majority compound thujanone | [113] |
| Sorghum | Osmolyte concentration, rates of gaseous exchange attributes, and antioxidant enzymatic activity | [114] |
| Soursop | Stomatal conductance, CO2 assimilation rate, transpiration, and carboxylation efficiency | [115] |
| Soybean | Leaf chlorophyll content index, anthocyanins content, leaf area, water use efficiency, seed filling duration, assimilate mobilization efficiency, and seed mass | [116] |
| Oil content per soybean seed, seed yield | ||
| Strawberry | Fresh and dry weight of shoots and roots, activity of ascorbate peroxidase, peroxidase, and superoxide dismutase enzymes | [117] |
| Antioxidant enzyme activities, ascorbic acid level | [118] | |
| Sunflower | Uptake of K+ ion | [119] |
| Tomato | Upregulation of ABA biosynthesis genes, zeaxanthin epoxidase, 9-cis-epoxycarotenoid dioxygenase, and aldehyde oxidases | [120] |
| Increase in the ascorbate-peroxidase and glutathione reductase activity | [121] | |
| Leaf concentration of sodium, proline, and soluble sugars | [122] | |
| Wheat | Net photosynthetic rate, transpiration rate, stomatal conductance, maximum quantum efficiency of PSII photochemistry, actual photochemical efficiency of PSII, electron transport rate, photochemical quenching coefficient, effective quantum yield of PSII photochemistry | [123] |
| Accelerating the metabolic flow, promoting nitrogen metabolism | ||
| ATP content and H+-pump activity of the roots | [124] | |
| Upregulation of the glyoxalase system and ascorbate–glutathione cycle |
| Plant Species | Target Areas of Beneficial Effect | References |
|---|---|---|
| Ammi visnaga | Two major secondary metabolite γ-pyrones, the khellin and visnagin content | [142] |
| Aristotelia chilensis | Phenol content, antioxidant capacity | [143] |
| Barley | Stem length, plant dry weights, chlorophyll concentration, relative water content, activity of antioxidant enzymes, and grain yield | [144] |
| Chinese cabbage | Superoxide dismutase, catalase, guaiacol peroxidase, and ascorbate peroxidase content, upregulation of pyrroline-5-carboxylate synthase genes (P5CSA and P5CSB) | [137] |
| Creeping bentgrass | Accumulation of amino acids (proline, serine, threonine, and alanine) and carbohydrates (glucose, mannose, fructose, and cellobiose) | [145] |
| Grape | Carotenoids content, CAT, APX, and GPX enzymes activities | [146] |
| Impatiens walleriana | Expression of the gene Δ1-pyrroline-5-carboxylate synthetase (P5CS) and Δ1-pyrroline-5-carboxylate reductase gene (P5CR) | [147] |
| Lemon verbena | Improving physiological parameters and essential oil content | [148] |
| Maize | Antioxidant enzymes activity, proline and soluble sugar content | [149] |
| Mentha pulegium | Relative water content, proline accumulation, antioxidant enzymes activities (SOD, POX, and PPO) | [90] |
| Mustard | Increasing the proline production through the increase in γ-glutamyl kinase (GK) and decrease in proline oxidase (PROX) activity | [93] |
| Water potential, osmotic potential, water use efficiency, photosynthetic nitrogen use efficiency | [150] | |
| Onion | Membrane stability index, relative water content | [151] |
| Pearl millet and wheat | Plant height and grain yield | [100] |
| Pumpkin | Carbohydrate and fatty acid content | [152] |
| Purslane | Photosynthetic pigments, gas exchanges, compatible solutes and secondary metabolites | [153] |
| Radish | Shoot mass, storage root mass, parameters of gas exchange | [154] |
| Rapeseed | Leaf ultra-structures | [87] |
| Rice | Photosynthetic pigments, proline content | [128] |
| Plant growth, dry weight, metabolism or metabolic activities, the nutritional status | [87] | |
| Rosemary | Essential oil content and quality | [155] |
| Ruta graveolens | Essential oil content and composition | [156] |
| Safflower | Non-enzymatic defense system (scavengers) | [157] |
| Rate of photosynthesis, anthocyanin content, phenylalanine ammonia lyase activity | [158] | |
| Sesame | Growth and various physiological processes | [159] |
| Net photosynthetic rate, stomatal conductance, leaf area index, chlorophyll a, b and total chlorophyll contents, maximum quantum efficiency of PSII, and plant dry matter and seed yield | [160] | |
| Osmoprotectant contents, antioxidant defense system, mineral nutrients in plant organs, photosynthesis | [161] | |
| Shallots | Leaf relative water content, membrane stability index, chlorophyll content, onion yield | [162] |
| Squash plant | Chlorophyll fluorescence, osmoprotectants | [163] |
| Strawberry | Total leaf area, leaf and shoot dry matter, catalase and peroxidase activity | [164] |
| Sunflower | Total chlorophyll and carotenoid content | [165] |
| Sweet basil | Growth parameters, photosynthetic pigments, and relative water content | [166] |
| Photosynthesis, antioxidant defense system | [167] | |
| Thyme | P-cymene oil content, rosmarinic acid content, total polyphenolic content | [168] |
| Thymol, carvacrol, linalool, p-cymene, and γ-terpinene content | [169] | |
| Tomato | Antioxidant enzyme activities, net photosynthetic rate and water use efficiency | [170] |
| Vicia faba | Maintaining an enhanced pool of reducing agents | [68] |
| Wheat | Membrane stability, total flavonoids and total phenol contents, CAT and SOD activities | [171] |
| Plant height, spike length, number of grains per spike, 1000 grain weight, chlorophyll content, relative water content | [172] | |
| Proline and soluble sugar content | [173] | |
| Carbon metabolism and signal transduction, energy production and protection in Lok1 | [174] | |
| Accumulation of soluble sugars, potassium, magnesium, and calcium | [175] | |
| Enzymatic and non-enzymatic antioxidant system | [176] |
| Plant Species | Heavy Metal | Target Areas of Beneficial Effect | References |
|---|---|---|---|
| Arabidopsis thaliana | Cadmium | Upregulation of electron transport-related and amylase-encoding genes | [189] |
| Bean | Cadmium | Superoxide dismutase, catalase, ascorbate peroxidase, and glutathione reductase | [190] |
| Nickel and lead | Carbonic anhydrase, nitrate reductase, catalase, peroxidase, superoxide dismutase, photosynthetic pigment, carbohydrate contents | [191] | |
| Flax | Cadmium | Levels of monogalactosyldiacylglycerol, phosphatidylglycerol, phosphatidic acid, and polyunsaturated fatty acids | [192] |
| Lemon balm | Mercury | Upregulation of chlorophyll synthase and phenylalanine ammonia-lyase genes as key components of chlorophyll and phenylpropanoid pathways | [193] |
| Maize | Copper | Seed metabolic activity, endogenous SA level, and carotenoid level | [194] |
| Lead | Protein and glutathione contents, nitrate reductase activity | [195] | |
| Arsenic | Ascorbate–glutathione cycle, glyoxalase system | [196] | |
| Chromium | Accumulations of osmolytes, antioxidants, and endogenous polyamines | [197] | |
| Melon | Cadmium | Chlorophyll content, photosynthetic activity, superoxide dismutase, guaiacol peroxidase, catalase, and ascorbate peroxidase activities, content of soluble protein and free proline | [198] |
| Mustard | Lead | Enzymatic and non-enzymatic antioxidant system | [199] |
| Peanut | Cadmium | Growth, chlorophyll content, photosynthesis, and mineral nutrition | [200] |
| Pepper | Lead | Growth parameters, biomass, leaf water status, and asa-GSH cycle-related enzyme activities | [201] |
| Potato | Cadmium | Relative water content, chlorophyll, proline, and endogenous SA contents | [202] |
| Rapeseed | Arsenic | Phytochelatin biosynthesis, S-assimilatory pathway, carbohydrate metabolism, rubisco, γ-glutamyl kinase, and proline oxidase enzyme activities | [203] |
| Rice | Arsenic | Endogenous levels of NO, nitrate reductase, and SA | [204] |
| Cadmium | Photochemical activity of both photosystems, electron flow, energy distribution between pigment–protein complexes, kinetic parameters of oxygen evolution reactions | [205] | |
| Restricting Cd uptake and accumulation | [206] | ||
| Ryegrass | Cadmium | Uptake and translocation of mineral elements | [207] |
| Tomato | Cadmium | Catalase activity | [208] |
| Wheat | Arsenic | Malondialdehyde content | [209] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decsi, K.; Ahmed, M.; Abdul-Hamid, D.; Tóth, Z. The Role of Salicylic Acid in Activating Plant Stress Responses—Results of the Past Decade and Future Perspectives. Int. J. Mol. Sci. 2025, 26, 4447. https://doi.org/10.3390/ijms26094447
Decsi K, Ahmed M, Abdul-Hamid D, Tóth Z. The Role of Salicylic Acid in Activating Plant Stress Responses—Results of the Past Decade and Future Perspectives. International Journal of Molecular Sciences. 2025; 26(9):4447. https://doi.org/10.3390/ijms26094447
Chicago/Turabian StyleDecsi, Kincső, Mostafa Ahmed, Donia Abdul-Hamid, and Zoltán Tóth. 2025. "The Role of Salicylic Acid in Activating Plant Stress Responses—Results of the Past Decade and Future Perspectives" International Journal of Molecular Sciences 26, no. 9: 4447. https://doi.org/10.3390/ijms26094447
APA StyleDecsi, K., Ahmed, M., Abdul-Hamid, D., & Tóth, Z. (2025). The Role of Salicylic Acid in Activating Plant Stress Responses—Results of the Past Decade and Future Perspectives. International Journal of Molecular Sciences, 26(9), 4447. https://doi.org/10.3390/ijms26094447






