Precise Electromagnetic Modulation of the Cell Cycle and Its Applications in Cancer Therapy
Abstract
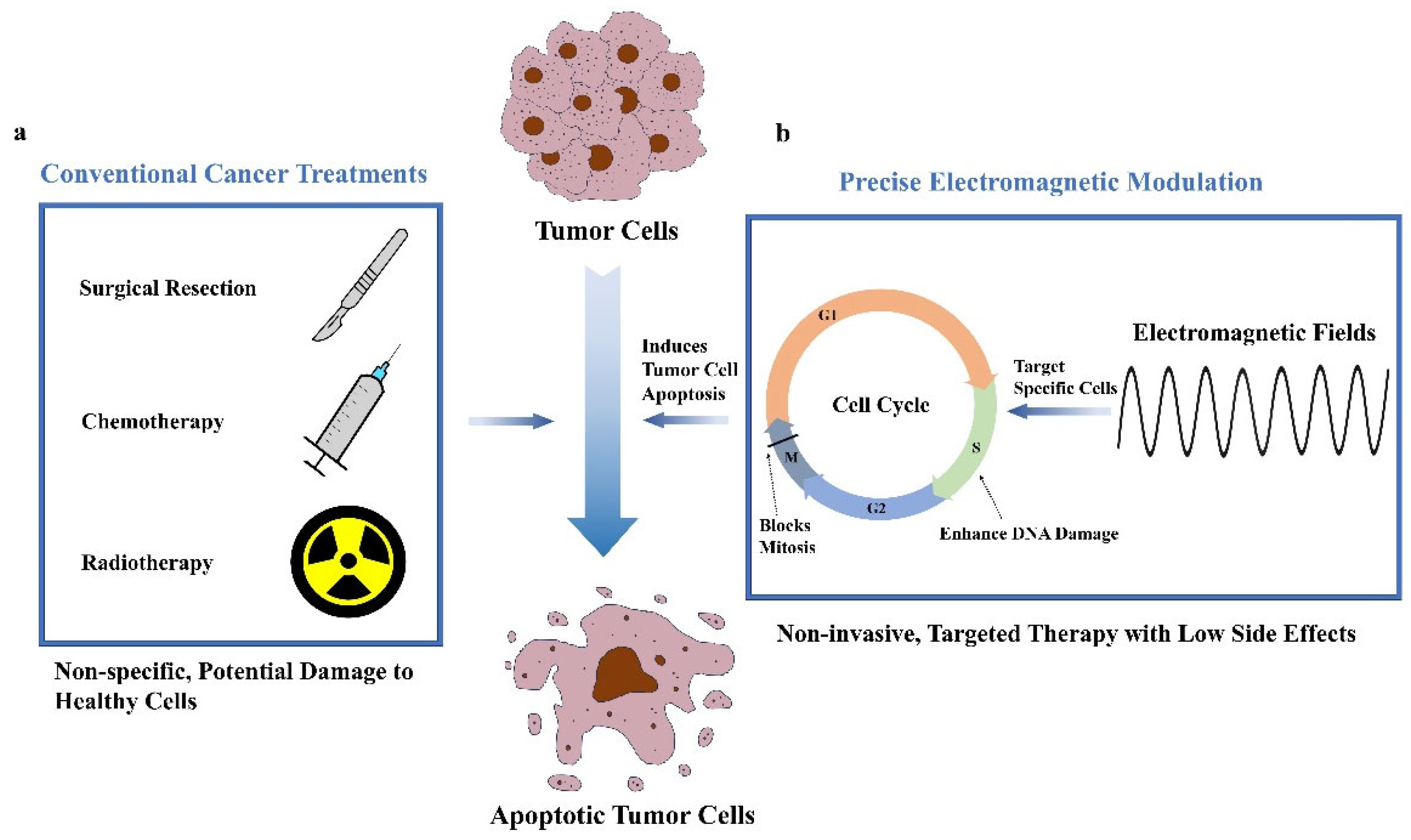
1. Introduction
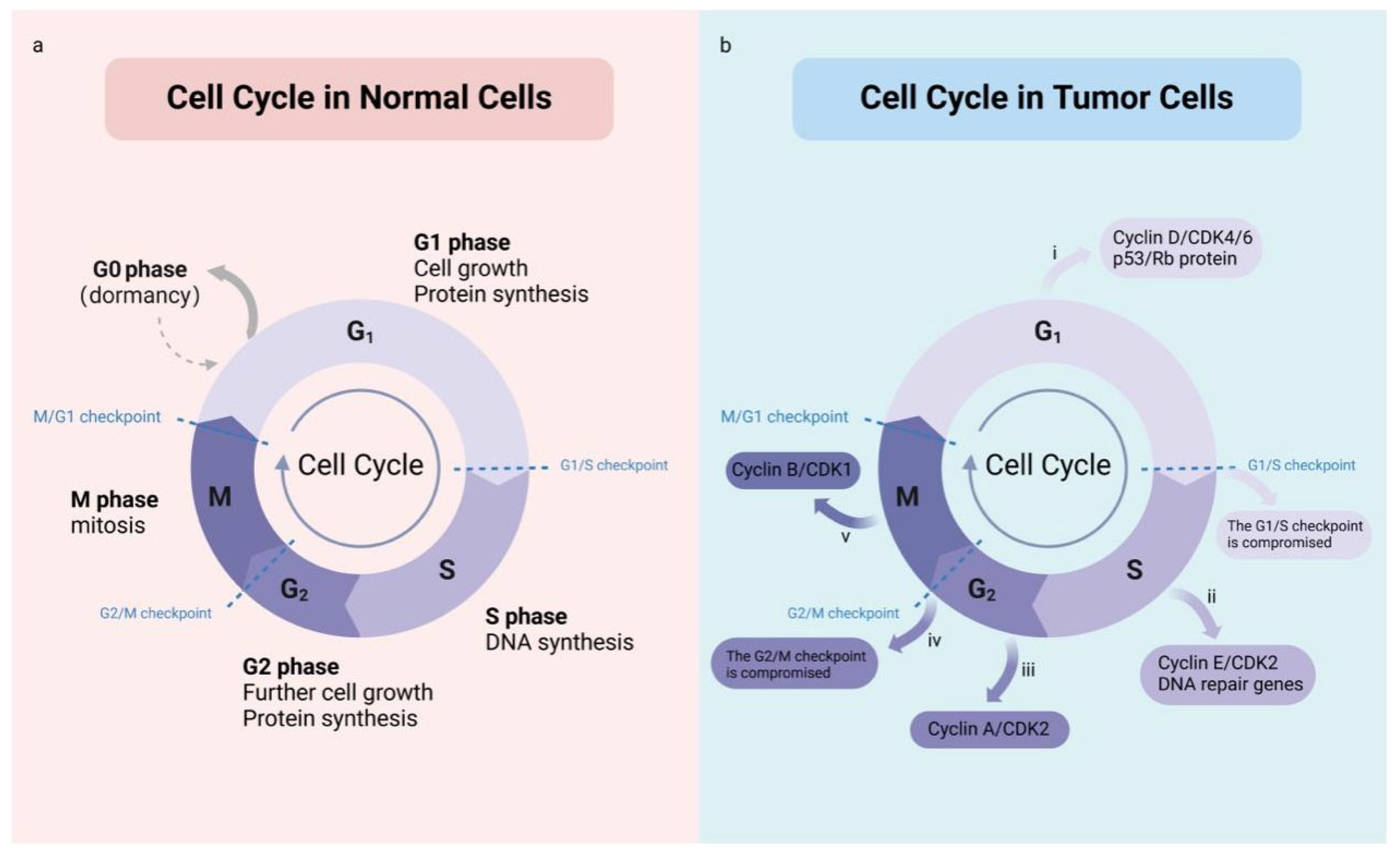
2. Cell Cycle and Tumor Growth
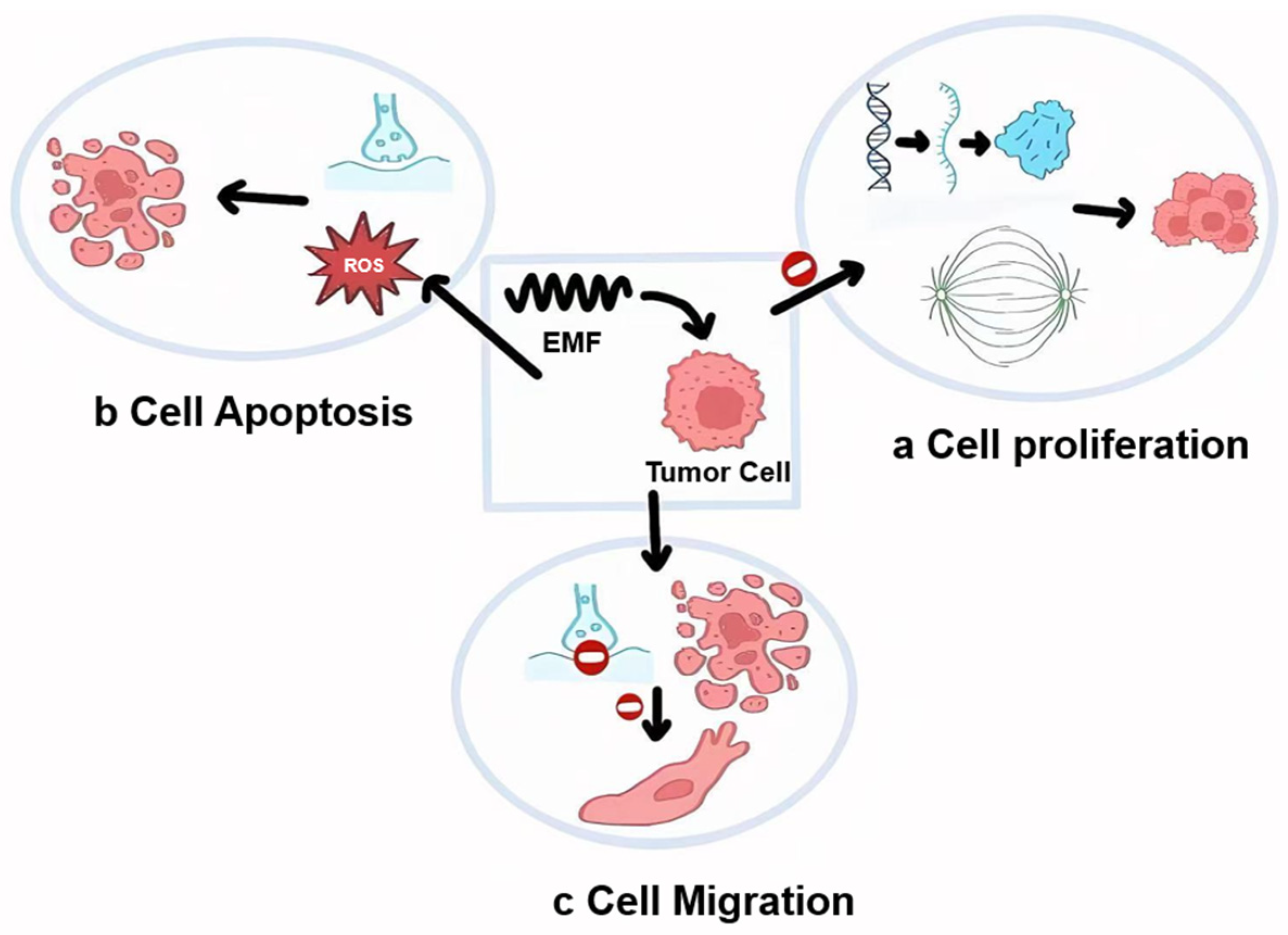
3. Electromagnetic Effects on Cells
4. The Effects of EMFs on Tumor Cell Dynamics and Synergistic Potential with Conventional Treatments
5. Precise Electromagnetic Regulation of the Cell Cycle
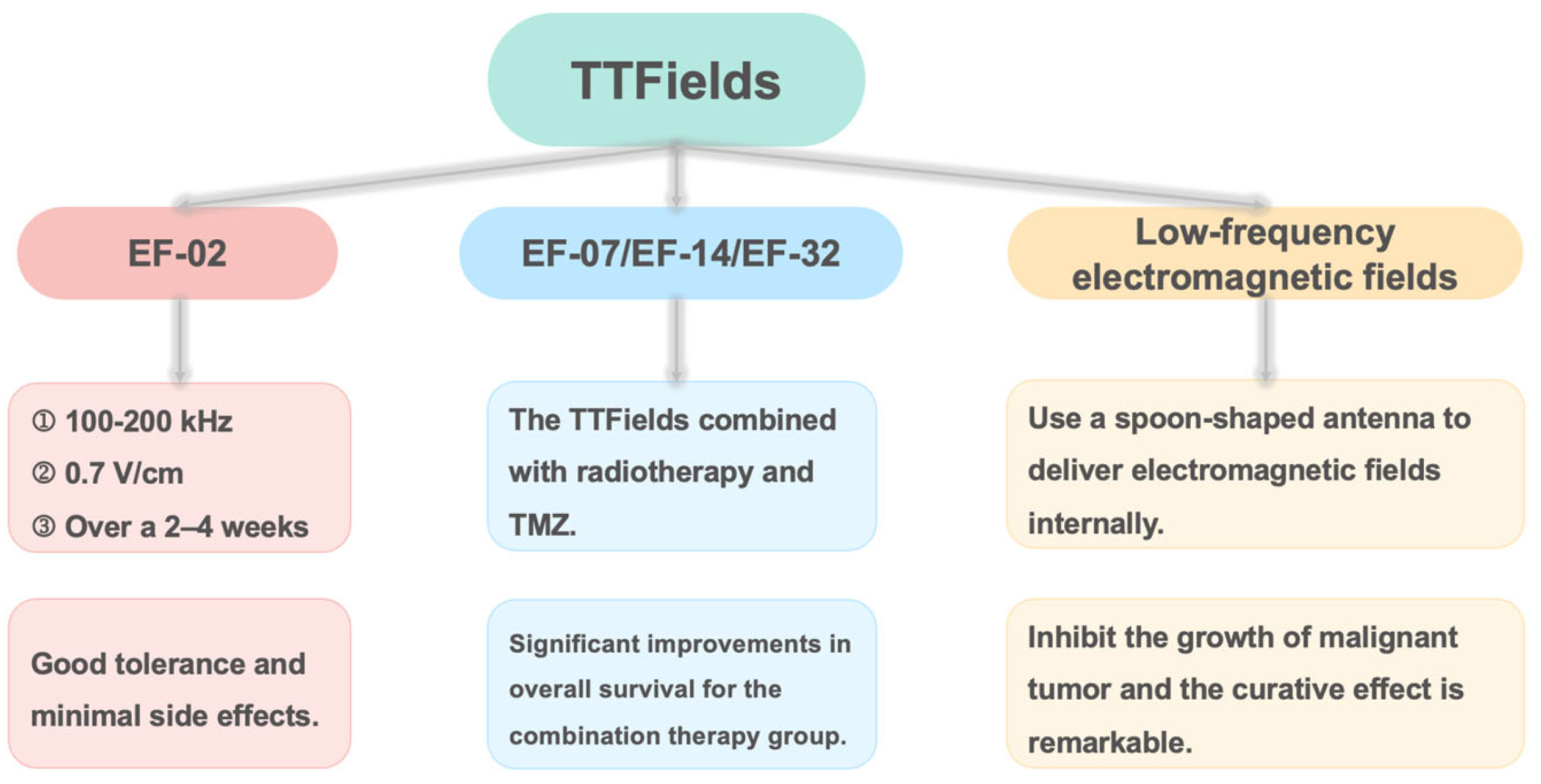
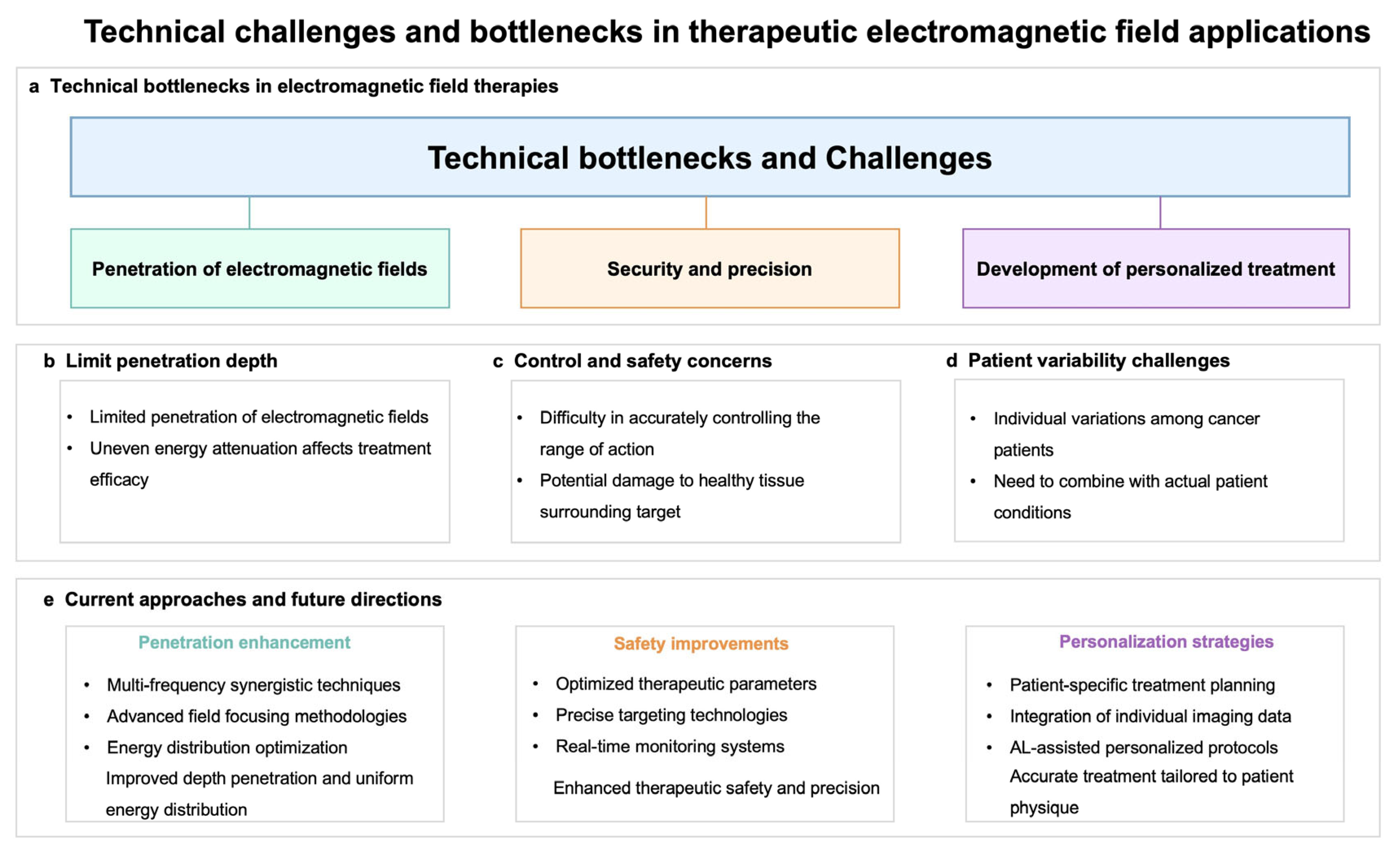
6. Application Cases and Challenges
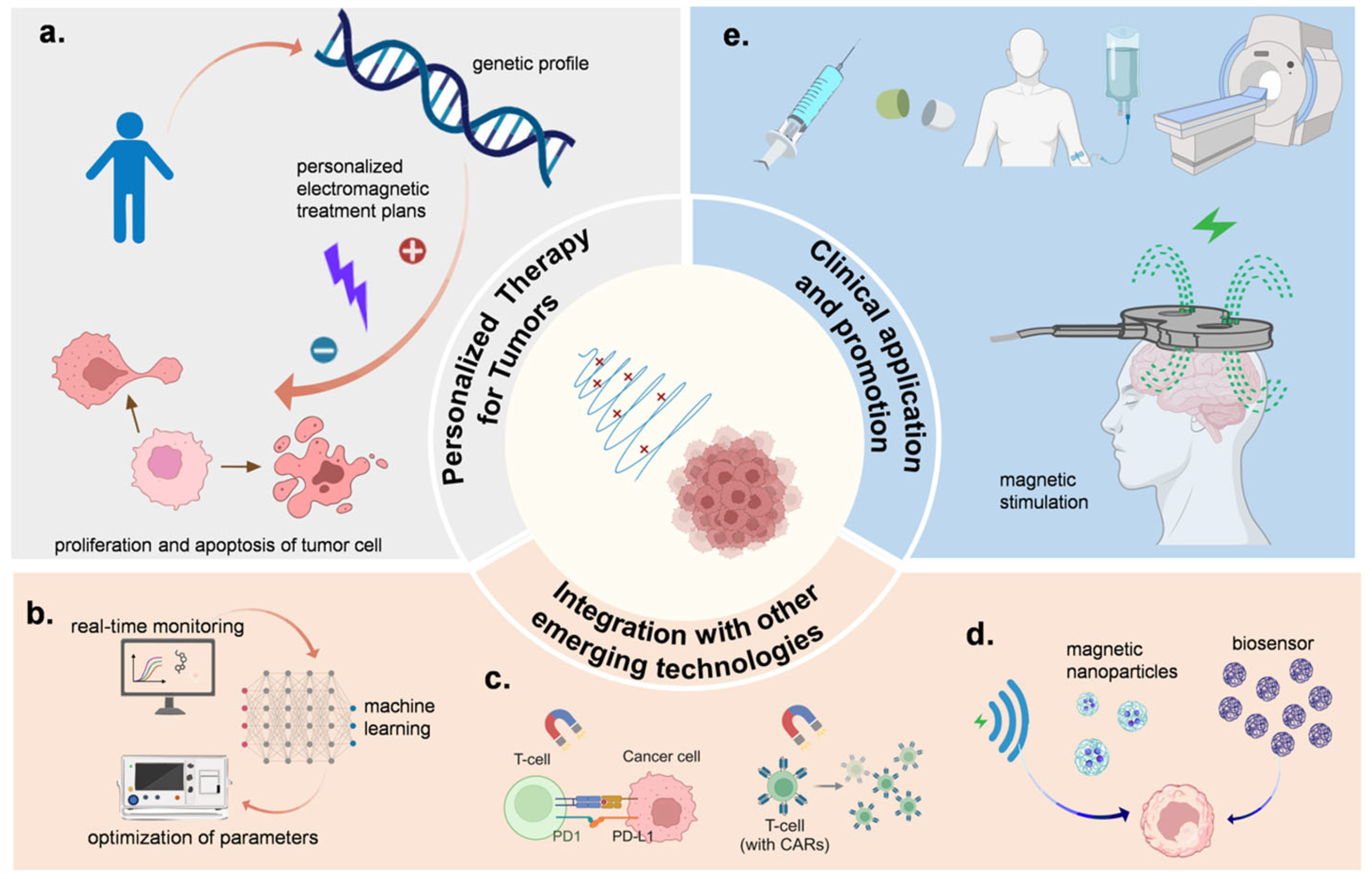
7. Future Directions of Electromagnetic Control Technology in Tumor Treatment
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| EM | Electromagnetic |
| G1 phase | First gap phase |
| S phase | Synthesis phase |
| G2 phase | Second gap phase |
| M phase | Mitosis phase |
| ROS | Reactive oxygen species |
| CKIs | Cyclin-Dependent Kinase Inhibitors |
| CDKs | Cyclin-Dependent Kinases |
| CDK4 | Cyclin-Dependent Kinase 4 |
| CDK6 | Cyclin-Dependent Kinase 6 |
| CDK2 | Cyclin-Dependent Kinase 2 |
| ATM | Ataxia Telangiectasia Mutated |
| ATR | Ataxia Telangiectasia and Rad3 Related |
| CHK1 | Checkpoint Kinase 1 |
| VGCCs | Voltage-gated calcium channels |
| EMF | Electromagnetic field |
| ELF | Extremely low-frequency |
| PMF | Pulsed low-frequency |
| TFF2 | Trefoil Factor 2 |
| VNS | Vagus nerve stimulation |
| MDSCs | Myeloid-derived suppressor cells |
| miRNA | microRNA |
| NSMF | Non-sinusoidal magnetic field |
| RoMEA | Relative oxygen metabolic efficiency assay |
| EIT | Electrical impedance tomography |
| BIS | Bioimpedance spectroscopy |
| HTS | High-throughput screening |
| GBM | Glioblastoma |
| TMZ | Temozolomide |
| PFS | Progression-free survival |
| OS | Overall survival |
| TTP | Time to progression |
| MF | Magnetic field |
| AI | Artificial intelligence |
| MNPs | Magnetic nanoparticles |
| RFA | Radiofrequency ablation |
| LIPUS | Low-intensity pulsed ultrasound |
| LIPUS | Low-intensity pulsed ultrasound |
| MWA | Microwave ablation |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Trayanova, N.A.; Popescu, D.M.; Shade, J.K. Machine Learning in Arrhythmia and Electrophysiology. Circ. Res. 2021, 128, 544–566. [Google Scholar] [CrossRef] [PubMed]
- Garces, A.H.I.; Porta, N.; Graham, T.A.; Banerji, U. Clinical trial designs for evaluating and exploiting cancer evolution. Cancer Treat. Rev. 2023, 118, 102583. [Google Scholar] [CrossRef] [PubMed]
- Kleinberg, L.; Sloan, L.; Grossman, S.; Lim, M. Radiotherapy, Lymphopenia, and Host Immune Capacity in Glioblastoma: A Potentially Actionable Toxicity Associated With Reduced Efficacy of Radiotherapy. Neurosurgery 2019, 85, 441–453. [Google Scholar] [CrossRef]
- Pathania, A.S.; Chava, H.; Balusu, R.; Pasupulati, A.K.; Coulter, D.W.; Challagundla, K.B. The crosstalk between non-coding RNAs and cell-cycle events: A new frontier in cancer therapy. Mol. Ther. Oncol. 2024, 32, 200785. [Google Scholar] [CrossRef]
- Diehl, F.F.; Sapp, K.M.; Heiden, M.G.V. The bidirectional relationship between metabolism and cell cycle control. Trends Cell Biol. 2024, 34, 136–149. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Q.; Lv, X.; Lin, T. Progressive Study on the Non-thermal Effects of Magnetic Field Therapy in Oncology. Front. Oncol. 2021, 11, 638146. [Google Scholar] [CrossRef]
- Huang, J.; Wang, L.Y.; Wang, B.; Liu, G.; Wang, W.C.; Ge, C.Q. Research Progress on Modulation of Electromagnetic Performance through Micro-nanostructure Design. J. Inorg. Mater. 2024, 39, 853–870. [Google Scholar] [CrossRef]
- Choi, Y.H. Tacrolimus Induces Apoptosis in Leukemia Jurkat Cells through Inactivation of the Reactive Oxygen Species-dependent Phosphoinositide-3-Kinase/Akt Signaling Pathway. Biotechnol. Bioprocess Eng. 2022, 27, 183–192. [Google Scholar] [CrossRef]
- Wolf, F.I.; Torsello, A.; Tedesco, B.; Fasanella, S.; Boninsegna, A.; D’Ascenzo, M.; Grassi, C.; Azzena, G.B.; Cittadini, A. 50-Hz extremely low frequency electromagnetic fields enhance cell proliferation and DNA damage: Possible involvement of a redox mechanism. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2005, 1743, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, J.; Pokorny, J.; Vrba, J. Generation of Electromagnetic Field by Microtubules. Int. J. Mol. Sci. 2021, 22, 8215. [Google Scholar] [CrossRef] [PubMed]
- Tuieng, R.J.; Cartmell, S.H.; Kirwan, C.C.; Sherratt, M.J. The Effects of Ionising and Non-Ionising Electromagnetic Radiation on Extracellular Matrix Proteins. Cells 2021, 10, 3041. [Google Scholar] [CrossRef]
- Berg, H.; GÜnther, B.; Hilger, I.; Radeva, M.; Traitcheva, N.; Wollweber, L. Bioelectromagnetic Field Effects on Cancer Cells and Mice Tumors. Electromagn. Biol. Med. 2010, 29, 132–143. [Google Scholar] [CrossRef]
- Wang, Z. Cell Cycle Progression and Synchronization: An Overview. In Methods in Molecular Biology; Springer: Clifton, NJ, USA, 2022; Volume 2579, pp. 3–23. [Google Scholar] [CrossRef]
- Gao, S.W.; Liu, F. Novel insights into cell cycle regulation of cell fate determination. J. Zhejiang Univ.-Sci. B 2019, 20, 467–475. [Google Scholar] [CrossRef]
- Yam, C.Q.X.; Lim, H.H.; Surana, U. DNA damage checkpoint execution and the rules of its disengagement. Front. Cell Dev. Biol. 2022, 10, 1020643. [Google Scholar] [CrossRef]
- Jamasbi, E.; Hamelian, M.; Hossain, M.A.; Varmira, K. The cell cycle, cancer development and therapy. Mol. Biol. Rep. 2022, 49, 10875–10883. [Google Scholar] [CrossRef]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef]
- Cersosimo, R.J. Cyclin-dependent kinase 4/6 inhibitors for the management of advanced or metastatic breast cancer in women. Am. J. Health-Syst. Pharm. 2019, 76, 1183–1202. [Google Scholar] [CrossRef]
- Desnoyers, A.; Nadler, M.B.; Kumar, V.; Saleh, R.; Amir, E. Comparison of treatment-related adverse events of different Cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: A network meta-analysis. Cancer Treat. Rev. 2020, 90, 102086. [Google Scholar] [CrossRef]
- Xia, P.; Liu, Y.N.; Chen, J.R.; Cheng, Z.K. Cell Cycle Proteins as Key Regulators of Postmitotic Cell Death. Yale J. Biol. Med. 2019, 92, 641–650. [Google Scholar] [PubMed]
- Liu, J.; Peng, Y.H.; Wei, W.Y. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022, 32, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.; Infante, J.R. Targeting CDK4/6 in patients with cancer. Cancer Treat. Rev. 2016, 45, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Shckorbatov, Y.G. Impact of electromagnetic radiation on human and animal cells: Approaches, results, perspectives. In Proceedings of the 2016 8th International Conference on Ultrawideband and Ultrashort Impulse Signals (UWBUSIS), Odessa, Ukraine, 5–11 September 2016; pp. 54–57. [Google Scholar]
- Tian, Z.; Yu, T.; Liu, J.; Wang, T.; Higuchi, A. Introduction to stem cells. Prog. Mol. Biol. Transl. Sci. 2023, 199, 3–32. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2020, 56, 15. [Google Scholar] [CrossRef]
- Zou, T.; Lin, Z. The Involvement of Ubiquitination Machinery in Cell Cycle Regulation and Cancer Progression. Int. J. Mol. Sci. 2021, 22, 5754. [Google Scholar] [CrossRef]
- Gupta, N.; Huang, T.T.; Horibata, S.; Lee, J.M. Cell cycle checkpoints and beyond: Exploiting the ATR/CHK1/WEE1 pathway for the treatment of PARP inhibitor-resistant cancer. Pharmacol. Res. 2022, 178, 106162. [Google Scholar] [CrossRef]
- Chu, C.; Geng, Y.; Zhou, Y.; Sicinski, P. Cyclin E in normal physiology and disease states. Trends Cell Biol. 2021, 31, 732–746. [Google Scholar] [CrossRef]
- Liang, H.Z.; Zhu, Y.; Zhao, Z.Y.; Du, J.T.; Yang, X.Y.; Fang, H.; Hou, X.B. Structure-Based Design of 2-Aminopurine Derivatives as CDK2 Inhibitors for Triple-Negative Breast Cancer. Front. Pharmacol. 2022, 13, 864342. [Google Scholar] [CrossRef]
- O’Leary, B.; Finn, R.S.; Turner, N.C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 2016, 13, 417–430. [Google Scholar] [CrossRef]
- Spring, L.M.; Wander, S.A.; Andre, F.; Moy, B.; Turner, N.C.; Bardia, A. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: Past, present, and future. Lancet 2020, 395, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.E.; Kovatcheva, M.; Davis, L.E.; Tap, W.D.; Koff, A. CDK4/6 Inhibitors: The Mechanism of Action May Not Be as Simple as Once Thought. Cancer Cell 2018, 34, 9–20. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, N.; Conklin, D.; Beckmann, R.; Luo, T.; Chau, K.; Thomas, J.; Mc Nulty, A.; Marchal, C.; Kalous, O.; von Euw, E.; et al. Preclinical Activity of Abemaciclib Alone or in Combination with Antimitotic and Targeted Therapies in Breast Cancer. Mol. Cancer Ther. 2018, 17, 897–907. [Google Scholar] [CrossRef]
- Patnaik, A.; Rosen, L.S.; Tolaney, S.M.; Tolcher, A.W.; Goldman, J.W.; Gandhi, L.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Hilton, J.F.; et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non-Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discov. 2016, 6, 740–753. [Google Scholar] [CrossRef]
- Chen, W.J.; Chang, C.Y.; Lin, J.K. Induction of G1 phase arrest in MCF human breast cancer cells by pentagalloylglucose through the down-regulation of CDK4 and CDK2 activities and up-regulation of the CDK inhibitors p27(Kip) and p21(Cip). Biochem. Pharmacol. 2003, 65, 1777–1785. [Google Scholar] [CrossRef]
- Gong, X.; Litchfield, L.M.; Webster, Y.; Chio, L.C.; Wong, S.S.; Stewart, T.R.; Dowless, M.; Dempsey, J.; Zeng, Y.; Torres, R.; et al. Genomic Aberrations that Activate D-type Cyclins Are Associated with Enhanced Sensitivity to the CDK4 and CDK6 Inhibitor Abemaciclib. Cancer Cell 2017, 32, 761–776.e6. [Google Scholar] [CrossRef]
- Song, G.M.; Liu, J.; Tang, X.; Zhong, J.; Zeng, Y.H.; Zhang, X.D.; Zhou, J.B.; Zhou, J.; Cao, L.; Zhang, Q.F.; et al. Cell cycle checkpoint revolution: Targeted therapies in the fight against malignant tumors. Front. Pharmacol. 2024, 15, 1459057. [Google Scholar] [CrossRef]
- Gholipour Hamedani, B.; Goliaei, B.; Shariatpanahi, S.P.; Nezamtaheri, M. An overview of the biological effects of extremely low frequency electromagnetic fields combined with ionizing radiation. Prog. Biophys. Mol. Biol. 2022, 172, 50–59. [Google Scholar] [CrossRef]
- Pophof, B.; Henschenmacher, B.; Kattnig, D.R.; Kuhne, J.; Vian, A.; Ziegelberger, G. Biological Effects of Electric, Magnetic, and Electromagnetic Fields from 0 to 100 MHz on Fauna and Flora: Workshop Report. Health Phys. 2023, 124, 39–52. [Google Scholar] [CrossRef]
- Levitt, B.B.; Lai, H.C.; Manville, A.M. Effects of non-ionizing electromagnetic fields on flora and fauna, part 1. Rising ambient EMF levels in the environment. Rev. Environ. Health 2022, 37, 81–122. [Google Scholar] [CrossRef]
- Sun, J.; Tong, Y.; Jia, Y.; Jia, X.; Wang, H.; Chen, Y.; Wu, J.; Jin, W.; Ma, Z.; Cao, K.; et al. Effects of extremely low frequency electromagnetic fields on the tumor cell inhibition and the possible mechanism. Sci. Rep. 2023, 13, 6989. [Google Scholar] [CrossRef] [PubMed]
- Ashdown, C.P.; Johns, S.C.; Aminov, E.; Unanian, M.; Connacher, W.; Friend, J.; Fuster, M.M. Pulsed Low-Frequency Magnetic Fields Induce Tumor Membrane Disruption and Altered Cell Viability. Biophys. J. 2020, 118, 1552–1563. [Google Scholar] [CrossRef]
- Cha, D.I.; Lee, M.W.; Jeong, W.K.; Ahn, S.H.; Kang, T.W.; Song, K.D.; Min, J.H.; Rhim, H.; Lim, H.K. Rim-arterial enhancing primary hepatic tumors with other targetoid appearance show early recurrence after radiofrequency ablation. Eur. Radiol. 2021, 31, 6555–6567. [Google Scholar] [CrossRef]
- Zhen, C.; Zhang, G.; Wang, S.; Wang, J.; Fang, Y.; Shang, P. Electromagnetic fields regulate iron metabolism in living organisms: A review of effects and mechanism. Prog. Biophys. Mol. Biol. 2024, 188, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Schuermann, D.; Mevissen, M. Manmade Electromagnetic Fields and Oxidative Stress-Biological Effects and Consequences for Health. Int. J. Mol. Sci. 2021, 22, 3772. [Google Scholar] [CrossRef]
- Kıvrak, E.G.; Yurt, K.K.; Kaplan, A.A.; Alkan, I.; Altun, G. Effects of electromagnetic fields exposure on the antioxidant defense system. J. Microsc. Ultrastruct. 2017, 5, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, O.; Ueno, S. Effects of radio frequency magnetic fields on iron release from cage proteins. Bioelectromagnetics 2009, 30, 336–342. [Google Scholar] [CrossRef]
- Consales, C.; Merla, C.; Marino, C.; Benassi, B. Electromagnetic fields, oxidative stress, and neurodegeneration. Int. J. Cell Biol. 2012, 2012, 683897. [Google Scholar] [CrossRef]
- Funk, R.H.; Monsees, T.; Ozkucur, N. Electromagnetic effects—From cell biology to medicine. Prog. Histochem. Cytochem. 2009, 43, 177–264. [Google Scholar] [CrossRef]
- Falone, S.; Santini, S., Jr.; Cordone, V.; Di Emidio, G.; Tatone, C.; Cacchio, M.; Amicarelli, F. Extremely Low-Frequency Magnetic Fields and Redox-Responsive Pathways Linked to Cancer Drug Resistance: Insights from Co-Exposure-Based In Vitro Studies. Front. Public Health 2018, 6, 33. [Google Scholar] [CrossRef]
- García-Minguillán, O.; Maestú, C. 30 Hz, Could It Be Part of a Window Frequency for Cellular Response? Int. J. Mol. Sci. 2021, 22, 3642. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Hunt, T.; Ericson, M. The Slowest Shared Resonance: A Review of Electromagnetic Field Oscillations Between Central and Peripheral Nervous Systems. Front. Hum. Neurosci. 2021, 15, 796455. [Google Scholar] [CrossRef] [PubMed]
- Hack, S.J.; Kinsey, L.J.; Beane, W.S. An Open Question: Is Non-Ionizing Radiation a Tool for Controlling Apoptosis-Induced Proliferation? Int. J. Mol. Sci. 2021, 22, 1159. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.; Darvishi, B.; Javidi, M.A.; Mohammadian, A.; Shariatpanahi, S.P.; Eisavand, M.R.; Madjid Ansari, A. Cellular stress response to extremely low-frequency electromagnetic fields (ELF-EMF): An explanation for controversial effects of ELF-EMF on apoptosis. Cell Prolif. 2021, 54, e13154. [Google Scholar] [CrossRef]
- Lai, H.; Levitt, B.B. Cellular and molecular effects of non-ionizing electromagnetic fields. Rev. Environ. Health 2024, 39, 519–529. [Google Scholar] [CrossRef]
- Parham, F.; Portier, C.J.; Chang, X.Q.; Mevissen, M. The Use of signal-Transduction and Metabolic Pathways to Predict Human Disease Targets from Electric and Magnetic Fields Using in vitro Data in Human Cell Lines. Front. Public Health 2016, 4, 00193. [Google Scholar] [CrossRef]
- Simkó, M. Cell type specific redox status is responsible for diverse electromagnetic field effects. Curr. Med. Chem. 2007, 14, 1141–1152. [Google Scholar] [CrossRef]
- Akbarnejad, Z.; Eskandary, H.; Dini, L.; Vergallo, C.; Nematollahi-Mahani, S.N.; Farsinejad, A.; Abadi, M.F.S.; Ahmadi, M. Cytotoxicity of temozolomide on human glioblastoma cells is enhanced by the concomitant exposure to an extremely low-frequency electromagnetic field (100Hz, 100G). Biomed. Pharmacother. 2017, 92, 254–264. [Google Scholar] [CrossRef]
- Mohamed, A.F.; Nasr, M.; Amer, M.E.; Abuamara, T.M.M.; Abd-Elhay, W.M.; Kaabo, H.F.; Matar, E.E.R.; El Moselhy, L.E.; Gomah, T.A.; Deban, M.A.F.; et al. Anticancer and antibacterial potentials induced post short-term exposure to electromagnetic field and silver nanoparticles and related pathological and genetic alterations: In vitro study. Infect. Agents Cancer 2022, 17, 4. [Google Scholar] [CrossRef]
- Bergandi, L.; Lucia, U.; Grisolia, G.; Salaroglio, I.C.; Gesmundo, I.; Granata, R.; Borchiellini, R.; Ponzetto, A.; Silvagno, F. Thermomagnetic Resonance Effect of the Extremely Low Frequency Electromagnetic Field on Three-Dimensional Cancer Models. Int. J. Mol. Sci. 2022, 23, 7955. [Google Scholar] [CrossRef]
- Wang, L.; Duan, Y.F.; Lu, S.J.; Sun, J.F. Magnetic Nanomaterials Mediate Electromagnetic Stimulations of Nerves for Applications in Stem Cell and Cancer Treatments. J. Funct. Biomater. 2023, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Erin, N.; Duymuş, O.; Oztürk, S.; Demir, N. Activation of vagus nerve by semapimod alters substance P levels and decreases breast cancer metastasis. Regul. Pept. 2012, 179, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Buckner, C.A.; Buckner, A.L.; Koren, S.A.; Persinger, M.A.; Lafrenie, R.M. Inhibition of cancer cell growth by exposure to a specific time-varying electromagnetic field involves T-type calcium channels. PLoS ONE 2015, 10, e0124136. [Google Scholar] [CrossRef]
- Markov, M.S.; Hazlewood, C.F. Electromagnetic field dosimetry for clinical application. Environmentalist 2009, 29, 161–168. [Google Scholar] [CrossRef]
- Kirson, E.D.; Dbalý, V.; Tovarys, F.; Vymazal, J.; Soustiel, J.F.; Itzhaki, A.; Mordechovich, D.; Steinberg-Shapira, S.; Gurvich, Z.; Schneiderman, R.; et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 10152–10157. [Google Scholar] [CrossRef]
- Mattsson, M.O.; Zeni, O.; Simkó, M.; Scarfì, M.R. Editorial: Effects of Combined EMF Exposures and Co-exposures. Front. Public Health 2018, 6, 230. [Google Scholar] [CrossRef]
- Parate, D.; Kadir, N.D.; Celik, C.; Lee, E.H.; Hui, J.H.P.; Franco-Obregón, A.; Yang, Z. Pulsed electromagnetic fields potentiate the paracrine function of mesenchymal stem cells for cartilage regeneration. Stem Cell Res. Ther. 2020, 11, 46. [Google Scholar] [CrossRef]
- Pall, M.L. Scientific evidence contradicts findings and assumptions of Canadian Safety Panel 6: Microwaves act through voltage-gated calcium channel activation to induce biological impacts at non-thermal levels, supporting a paradigm shift for microwave/lower frequency electromagnetic field action. Rev. Environ. Health 2015, 30, 99–116. [Google Scholar] [CrossRef]
- Yakymenko, I.; Tsybulin, O.; Sidorik, E.; Henshel, D.; Kyrylenko, O.; Kyrylenko, S. Oxidative mechanisms of biological activity of low-intensity radiofrequency radiation. Electromagn. Biol. Med. 2016, 35, 186–202. [Google Scholar] [CrossRef]
- Blank, M.; Goodman, R. Electromagnetic fields stress living cells. Pathophysiology 2009, 16, 71–78. [Google Scholar] [CrossRef]
- Levin, M. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 2014, 25, 3835–3850. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.R.; Ding, J.; Dai, M.Y.; Liu, L.; Fang, Q.; Wang, D.W.; Wu, L.J.; Wang, Y. Insights Into Platelet-Derived MicroRNAs in Cardiovascular and Oncologic Diseases: Potential Predictor and Therapeutic Target. Front. Cardiovasc. Med. 2022, 9, 879351. [Google Scholar] [CrossRef] [PubMed]
- Shayeghan, M.; Forouzesh, F.; Ansari, A.M.; Javidi, M.A. DNMT1 and miRNAs: Possible epigenetics footprints in electromagnetic fields utilization in oncology. Med. Oncol. 2021, 38, 125. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Li, N.; Cao, Y.; Zhu, Y. Natural static magnetic field-induced apoptosis in liver cancer cell. Electromagn. Biol. Med. 2014, 33, 47–50. [Google Scholar] [CrossRef]
- Dai, W.; Qiao, X.; Fang, Y.; Guo, R.; Bai, P.; Liu, S.; Li, T.; Jiang, Y.; Wei, S.; Na, Z.; et al. Epigenetics-targeted drugs: Current paradigms and future challenges. Signal Transduct. Target. Ther. 2024, 9, 332. [Google Scholar] [CrossRef]
- Saliev, T.; Begimbetova, D.; Masoud, A.-R.; Matkarimov, B. Biological effects of non-ionizing electromagnetic fields: Two sides of a coin. Prog. Biophys. Mol. Biol. 2019, 141, 25–36. [Google Scholar] [CrossRef]
- Kelly, A.D.; Issa, J.J. The promise of epigenetic therapy: Reprogramming the cancer epigenome. Curr. Opin. Genet. Dev. 2017, 42, 68–77. [Google Scholar] [CrossRef]
- Jin, N.; George, T.L.; Otterson, G.A.; Verschraegen, C.; Wen, H.; Carbone, D.; Herman, J.; Bertino, E.M.; He, K. Advances in epigenetic therapeutics with focus on solid tumors. Clin. Epigenet. 2021, 13, 83. [Google Scholar] [CrossRef]
- Zimmerman, J.W.; Pennison, M.J.; Brezovich, I.; Yi, N.; Yang, C.T.; Ramaker, R.; Absher, D.; Myers, R.M.; Kuster, N.; Costa, F.P.; et al. Cancer cell proliferation is inhibited by specific modulation frequencies. Br. J. Cancer 2012, 106, 307–313. [Google Scholar] [CrossRef]
- Mun, E.J.; Babiker, H.M.; Weinberg, U.; Kirson, E.D.; Von Hoff, D.D. Tumor-Treating Fields: A Fourth Modality in Cancer Treatment. Clin. Cancer Res. 2018, 24, 266–275. [Google Scholar] [CrossRef]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.H.; Choi, B.I.; de Baère, T.; Dodd, G.D., 3rd; et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria—A 10-year update. J. Vasc. Interv. Radiol. 2014, 25, 1691–1705.e4. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.K.; Sehgal, C.M. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med. Biol. 2015, 41, 905–928. [Google Scholar] [CrossRef] [PubMed]
- Jooyan, N.; Goliaei, B.; Bigdeli, B.; Faraji-Dana, R.; Zamani, A.; Entezami, M.; Mortazavi, S.M.J. Direct and indirect effects of exposure to 900 MHz GSM radiofrequency electromagnetic fields on CHO cell line: Evidence of bystander effect by non-ionizing radiation. Environ. Res. 2019, 174, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.Q.; Wang, C.; Lu, D.F.; Zhao, X.D.; Tan, L.H.; Chen, X. Induction of apoptosis and ferroptosis by a tumor suppressing magnetic field through ROS-mediated DNA damage. Aging 2020, 12, 3662–3681. [Google Scholar] [CrossRef]
- Amirinejad, M.; Eftekhar-Vaghefi, S.H.; Mahani, S.N.N.; Salari, M.; Yahyapour, R.; Ahmadi-Zeidabadi, M. Exposure to Low-Frequency Radiation Changes the Expression of Nestin, VEGF, BCRP and Apoptosis Markers During Glioma Treatment Strategy: An In Vitro Study. Curr. Radiopharm. 2024, 17, 55–67. [Google Scholar] [CrossRef]
- Ma, T.; Ding, Q.; Liu, C.; Wu, H. Electromagnetic fields regulate calcium-mediated cell fate of stem cells: Osteogenesis, chondrogenesis and apoptosis. Stem Cell Res. Ther. 2023, 14, 133. [Google Scholar] [CrossRef]
- Nezamtaheri, M.S.; Goliaei, B.; Shariatpanahi, S.P.; Ansari, A.M. Differential biological responses of adherent and non-adherent (cancer and non-cancerous) cells to variable extremely low frequency magnetic fields. Sci. Rep. 2022, 12, 14225. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Xu, H.; Yang, Y.; Li, W.; Wu, H.; Liu, C. Extremely low frequency electromagnetic fields promote mesenchymal stem cell migration by increasing intracellular Ca(2+) and activating the FAK/Rho GTPases signaling pathways in vitro. Stem Cell Res. Ther. 2018, 9, 143. [Google Scholar] [CrossRef]
- Moori, M.; Norouzian, D.; Yaghmaei, P.; Farahmand, L. Electromagnetic field as a possible inhibitor of tumor invasion by declining E-cadherin/N-cadherin switching in triple negative breast cancer. Electromagn. Biol. Med. 2024, 43, 236–245. [Google Scholar] [CrossRef]
- Tanzhu, G.; Chen, L.; Xiao, G.; Shi, W.; Peng, H.; Chen, D.; Zhou, R. The schemes, mechanisms and molecular pathway changes of Tumor Treating Fields (TTFields) alone or in combination with radiotherapy and chemotherapy. Cell Death Discov. 2022, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, X.; Liu, Y.; Zhang, S.; Yu, T.; Chai, X.; He, J.; Yin, D.-C.; Zhang, C.-Y. The effect of magnetic fields on tumor occurrence and progression: Recent advances. Prog. Biophys. Mol. Biol. 2023, 179, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Alshahat, M.A.; Elgenedy, M.A.; Aboushady, A.A.; Williams, M.T.S. Cancer Treatment: An Overview of Pulsed Electric Field Utilization and Generation. Appl. Sci. 2023, 13, 10029. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Soltani, M.; Souri, M. Improving tumor treatment through intratumoral injection of drug-loaded magnetic nanoparticles and low-intensity ultrasound. Sci. Rep. 2024, 14, 1452. [Google Scholar] [CrossRef]
- Obrador, E.; Jihad-Jebbar, A.; Salvador-Palmer, R.; López-Blanch, R.; Oriol-Caballo, M.; Moreno-Murciano, M.P.; Navarro, E.A.; Cibrian, R.; Estrela, J.M. Externally Applied Electromagnetic Fields and Hyperthermia Irreversibly Damage Cancer Cells. Cancers 2023, 15, 3413. [Google Scholar] [CrossRef]
- Salinas-Asensio, M.M.; Ríos-Arrabal, S.; Artacho-Cordón, F.; Olivares-Urbano, M.A.; Calvente, I.; León, J.; Núñez, M.I. Exploring the radiosensitizing potential of magnetotherapy: A pilot study in breast cancer cells. Int. J. Radiat. Biol. 2019, 95, 1337–1345. [Google Scholar] [CrossRef]
- Yadegari Dehkordi, S.; Firoozabadi, S.M.; Forouzandeh Moghadam, M.; Shankayi, Z. Endocytosis induction by high-pulsed magnetic fields to overcome cell membrane barrier and improve chemotherapy efficiency. Electromagn. Biol. Med. 2021, 40, 438–445. [Google Scholar] [CrossRef]
- Homami, E.; Goliaei, B.; Shariatpanahi, S.P.; Habibi-Kelishomi, Z. Alternating electric fields can improve chemotherapy treatment efficacy in blood cancer cell U937 (non-adherent cells). BMC Cancer 2023, 23, 861. [Google Scholar] [CrossRef]
- Tota, M.; Jonderko, L.; Witek, J.; Novickij, V.; Kulbacka, J. Cellular and Molecular Effects of Magnetic Fields. Int. J. Mol. Sci. 2024, 25, 8973. [Google Scholar] [CrossRef]
- Fuster, M.M. Integrating electromagnetic cancer stress with immunotherapy: A therapeutic paradigm. Front. Oncol. 2024, 14, 1417621. [Google Scholar] [CrossRef]
- Messenheimer, D.J.; Jensen, S.M.; Afentoulis, M.E.; Wegmann, K.W.; Feng, Z.; Friedman, D.J.; Gough, M.J.; Urba, W.J.; Fox, B.A. Timing of PD-1 Blockade Is Critical to Effective Combination Immunotherapy with Anti-OX40. Clin. Cancer Res. 2017, 23, 6165–6177. [Google Scholar] [CrossRef] [PubMed]
- Lucia, U.; Grisolia, G. Seebeck-Peltier Transition Approach to Oncogenesis. Appl. Sci. 2020, 10, 7166. [Google Scholar] [CrossRef]
- Gottschalk, B.; Koshenov, Z.; Malli, R.; Graier, W.F. Implications of mitochondrial membrane potential gradients on signaling and ATP production analyzed by correlative multi-parameter microscopy. Sci. Rep. 2024, 14, 14784. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska-Olejniczak, K.; Bednarczyk, P. Novel insights into the role of ion channels in cellular DNA damage response. Mutat. Res.-Rev. Mutat. Res. 2024, 793, 108488. [Google Scholar] [CrossRef]
- Yang, T.R.; Huang, D.; Li, C.H.; Zhao, D.Y.; Li, J.S.; Zhang, M.J.; Chen, Y.F.; Wang, Q.N.; Liang, Z.C.; Liang, X.J.; et al. Rolling microneedle electrode array (RoMEA) empowered nucleic acid delivery and cancer immunotherapy. Nano Today 2021, 36, 101017. [Google Scholar] [CrossRef]
- Ihsan, M.F.; Kawashima, D.; Li, S.S.; Ogasawara, S.; Murata, T.; Takei, M. Non-invasive hERG channel screening based on electrical impedance tomography and extracellular voltage activation (EIT-EVA). Lab A Chip 2024, 24, 3183–3190. [Google Scholar] [CrossRef]
- Oh, T.I.; Kang, M.J.; Jeong, Y.J.; Zhang, T.; Yeo, S.G.; Park, D.C. Tissue Characterization Using an Electrical Bioimpedance Spectroscopy-Based Multi-Electrode Probe to Screen for Cervical Intraepithelial Neoplasia. Diagnostics 2021, 11, 2354. [Google Scholar] [CrossRef]
- Ramaswamy, V.D.; Keidar, M. Personalized Plasma Medicine for Cancer: Transforming Treatment Strategies with Mathematical Modeling and Machine Learning Approaches. Appl. Sci. 2024, 14, 355. [Google Scholar] [CrossRef]
- Hernández-Bule, M.L.; Medel, E.; Colastra, C.; Roldán, R.; Ubeda, A. Response of neuroblastoma cells to RF currents as a function of the signal frequency. BMC Cancer 2019, 19, 889. [Google Scholar] [CrossRef]
- Besler, E.; Wang, Y.C.; Sahakian, A.V. Early and Late Fusion Machine Learning on Multi-Frequency Electrical Impedance Data to Improve Radiofrequency Ablation Monitoring. IEEE J. Biomed. Health Inform. 2020, 24, 2359–2367. [Google Scholar] [CrossRef]
- Grubb, L.M.; Caliari, R.S. Fabrication approaches for high-throughput and biomimetic disease modeling. Acta Biomater. 2021, 132, 52–82. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Fang, Y.; Wang, R.; Liang, S.S. High-throughput solutions in tumor organoids: From culture to drug screening. Stem Cells 2024, 43, sxae070. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Nagatoishi, S.; Tsumoto, K.; Furukawa, Y. Discovery of chemical probes that suppress Wnt/β-catenin signaling through high-throughput screening. Cancer Sci. 2020, 111, 783–794. [Google Scholar] [CrossRef]
- Vercauteren, S.; Fiesack, S.; Maroc, L.; Verstraeten, N.; Dewachter, L.; Michiels, J.; Vonesch, S.C. The rise and future of CRISPR-based approaches for high-throughput genomics. FEMS Microbiol. Rev. 2024, 48, fuae020. [Google Scholar] [CrossRef]
- Huang, Y.; Shang, M.Q.; Liu, T.T.; Wang, K.J. High-throughput methods for genome editing: The more the better. Plant Physiol. 2022, 188, 1731–1745. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Lu, M. Advances in magnetic induction hyperthermia. Front. Bioeng. Biotechnol. 2024, 12, 1432189. [Google Scholar] [CrossRef]
- Singh, S.; Repaka, R. Parametric sensitivity analysis of critical factors affecting the thermal damage during RFA of breast tumor. Int. J. Therm. Sci. 2018, 124, 366–374. [Google Scholar] [CrossRef]
- Baik, J.; Lee, S.; Yang, S.; Park, S.M. Modularized Electrosurgical System With a Hybrid CPU-FPGA Chip for Real-Time Thermal Lesion Approximation. IEEE Trans. Instrum. Meas. 2022, 71, 4003210. [Google Scholar] [CrossRef]
- Chai, Z.P.; Lyu, L.X.; Pu, M.H.; Chen, X.W.; Zhu, J.Q.; Liang, H.G.; Ding, H.; Wu, Z.G. An Individually Controlled Multitined Expandable Electrode Using Active Cannula-Based Shape Morphing for On-Demand Conformal Radiofrequency Ablation Lesions. Adv. Intell. Syst. 2022, 4, 2100262. [Google Scholar] [CrossRef]
- Yu, A.; Zeng, J.; Yu, J.H.; Cao, S.; Li, A.L. Theory and application of TTFields in newly diagnosed glioblastoma. Cns Neurosci. Ther. 2024, 30, e14563. [Google Scholar] [CrossRef]
- Grosu, A.; Touchefeu, Y.; Brunner, T.; Gkika, E.; Thimme, R.; Cubillo, A. phase 2 HEPANOVA study of tumor treating fields (TTFields, 150 kHz) concomitant with sorafenib in advanced hepatocellular carcinoma (HCC): Interim safety analysis. Ann. Oncol. 2020, 31, S160. [Google Scholar] [CrossRef]
- Moser, J.C.; Salvador, E.; Deniz, K.; Swanson, K.; Tuszynski, J.; Carlson, K.W.; Karanam, N.K.; Patel, C.B.; Story, M.; Lou, E.M.; et al. The Mechanisms of Action of Tumor Treating Fields. Cancer Res. 2022, 82, 3650–3658. [Google Scholar] [CrossRef] [PubMed]
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S.J. Tumour treating fields therapy for glioblastoma: Current advances and future directions. Br. J. Cancer 2021, 124, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Salzberg, M.; Kirson, E.; Palti, Y.; Rochlitz, C. A Pilot Study with Very Low-Intensity, Intermediate-Frequency Electric Fields in Patients with Locally Advanced and/or Metastatic Solid Tumors. Onkologie 2008, 31, 362–365. [Google Scholar] [CrossRef]
- Guo, X.; Yang, X.; Wu, J.; Yang, H.; Li, Y.; Li, J.; Liu, Q.; Wu, C.; Xing, H.; Liu, P.; et al. Tumor-Treating Fields in Glioblastomas: Past, Present, and Future. Cancers 2022, 14, 3669. [Google Scholar] [CrossRef]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef]
- Kirson, E.D.; Schneiderman, R.S.; Dbaly, V.; Tovarys, F.; Vymazal, J.; Itzhaki, A.; Mordechovich, D.; Gurvich, Z.; Shmueli, E.; Goldsher, D.; et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med. Phys. 2009, 9, 1. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma A Randomized Clinical Trial. JAMA-J. Am. Med. Assoc. 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Kesari, S.; Ram, Z.; Investigators, E.F.T. Tumor-treating fields plus chemotherapy versus chemotherapy alone for glioblastoma at first recurrence: A post hoc analysis of the EF-14 trial. CNS Oncol. 2017, 6, 185–193. [Google Scholar] [CrossRef]
- Bähr, O.; Tabatabai, G.; Fietkau, R.; Goldbrunner, R.; Glas, M. Tumor treating fields (TTFields) therapy in patients with glioblastoma: Long-term survival results from TTFields in Germany in routine clinical care (TIGER) study. J. Clin. Oncol. 2024, 42, 2036. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Smeets, B.; Nijs, S.; Hoekstra, H. Infection after fracture fixation of the tibia: Analysis of healthcare utilization and related costs. Inj.-Int. J. Care Inj. 2017, 48, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Ceresoli, G.L.; Aerts, J.G.; Dziadziuszko, R.; Ramlau, R.; Cedres, S.; van Meerbeeck, J.P.; Mencoboni, M.; Planchard, D.; Chella, A.; Crinò, L.; et al. Tumour Treating Fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): A multicentre, single-arm phase 2 trial. Lancet Oncol. 2019, 20, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Ning, J.; Chen, L.; Zeng, Y.; Shi, Y.; Xiao, G.; He, S.; Tanzhu, G.; Zhou, R. Cost-effectiveness of tumor-treating fields plus standard therapy for advanced non-small cell lung cancer progressed after platinum-based therapy in the United States. Front. Pharmacol. 2024, 15, 1333128. [Google Scholar] [CrossRef]
- Rivera, F.; Benavides, M.; Gallego, J.; Guillen-Ponce, C.; Lopez-Martin, J.; Küng, M. Tumor treating fields in combination with gemcitabine or gemcitabine plus nab-paclitaxel in pancreatic cancer: Results of the PANOVA phase 2 study. Pancreatology 2019, 19, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Davidi, S.; Jacobovitch, S.; Shteingauz, A.; Martinez-Conde, A.; Braten, O.; Tempel-Brami, C.; Zeevi, E.; Frechtel-Gerzi, R.; Ene, H.; Dor-On, E.; et al. Tumor Treating Fields (TTFields) Concomitant with Sorafenib Inhibit Hepatocellular Carcinoma In Vitro and In Vivo. Cancers 2022, 14, 2959. [Google Scholar] [CrossRef]
- Neuhaus, E.; Zirjacks, L.; Ganser, K.; Klumpp, L.; Schüler, U.; Zips, D.; Eckert, F.; Huber, S.M. Alternating Electric Fields (TTFields) Activate Cav1.2 Channels in Human Glioblastoma Cells. Cancers 2019, 11, 110. [Google Scholar] [CrossRef]
- Chen, A.B.; Kotecha, R.; Chaney, M.; Balestrieri, K.; Gabrail, N.; Langer, C. The Keynote B36 (EF-36) Pilot Study of Tumor Treating Fields (TTFields) Therapy with pembrolizumab as First-Line Treatment of PD-L1-Positive, Advanced or Metastatic Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, e8. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Anadkat, M.J.; Ballo, M.T.; Iwamoto, F.; Jeyapalan, S.A.; La Rocca, R.V.; Schwartz, M.; Serventi, J.N.; Glas, M. Prevention and Management of Dermatologic Adverse Events Associated With Tumor Treating Fields in Patients With Glioblastoma. Front. Oncol. 2020, 10, 1045. [Google Scholar] [CrossRef]
- Karanam, N.K.; Srinivasan, K.; Ding, L.; Sishc, B.; Saha, D.; Story, M.D. Tumor-treating fields elicit a conditional vulnerability to ionizing radiation via the downregulation of BRCA1 signaling and reduced DNA double-strand break repair capacity in non-small cell lung cancer cell lines. Cell Death Dis. 2017, 8, e2711. [Google Scholar] [CrossRef]
- Ballo, M.T.; Urman, N.; Lavy-Shahaf, G.; Grewal, J.; Bomzon, Z.; Toms, S. Correlation of Tumor Treating Fields Dosimetry to Survival Outcomes in Newly Diagnosed Glioblastoma: A Large-Scale Numerical Simulation-Based Analysis of Data from the Phase 3 EF-14 Randomized Trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1106–1113. [Google Scholar] [CrossRef]
- Mittal, S.; Klinger, N.V.; Michelhaugh, S.K.; Barger, G.R.; Pannullo, S.C.; Juhász, C. Alternating electric tumor treating fields for treatment of glioblastoma: Rationale, preclinical, and clinical studies. J. Neurosurg. 2018, 128, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, J.; Ren, J.; Peng, J.; Zhong, R.; He, J.; Xu, T.; Yu, Z.; Jin, H.; Hao, S.; et al. Minimally-invasive implantable device enhances brain cancer suppression. EMBO Mol. Med. 2024, 16, 1704–1716. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, X.; Liu, Y.; Ouyang, B.; Zhang, J.; Jin, H.; Yu, Z.; Liu, R.; Li, Z.; Jiang, L.; et al. An implantable ultrasound-powered device for the treatment of brain cancer using electromagnetic fields. Sci. Adv. 2022, 8, eabm5023. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Hasegawa, K.; Izumi, N.; Kudo, M.; Shimada, M.; Yamanaka, N.; Inomata, M.; Kaneko, S.; Nakayama, H.; Kawaguchi, Y.; et al. Surgery versus Radiofrequency Ablation for Small Hepatocellular Carcinoma: A Randomized Controlled Trial (SURF Trial). Liver Cancer 2022, 11, 209–218. [Google Scholar] [CrossRef]
- Simon, C.J.; Dupuy, D.E.; DiPetrillo, T.A.; Safran, H.P.; Grieco, C.A.; Ng, T.; Mayo-Smith, W.W. Pulmonary radiofrequency ablation: Long-term safety and efficacy in 153 patients. Radiology 2007, 243, 268–275. [Google Scholar] [CrossRef]
- Cui, R.; Yu, J.; Kuang, M.; Duan, F.; Liang, P. Microwave ablation versus other interventions for hepatocellular carcinoma: A systematic review and meta-analysis. J. Cancer Res. Ther. 2020, 16, 379–386. [Google Scholar] [CrossRef]
- Liu, X.; Zhan, Y.; Wang, H.; Tang, X.; Cheng, Y. Radiofrequency ablation versus microwave ablation for lung cancer/lung metastases: A meta-analysis. ANZ J. Surg. 2025, 95, 56–65. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Overgaard, J.; Ccm Hulshof, M.; Dahl, O.; Arcangeli, G. ESHO 1–85. Hyperthermia as an adjuvant to radiation therapy in the treatment of locally advanced breast carcinoma. A randomized multicenter study by the European Society for Hyperthermic Oncology. Radiother. Oncol. 2024, 196, 110313. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic hyperthermia therapy for the treatment of glioblastoma: A review of the therapy’s history, efficacy and application in humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yang, Z.; Yu, H.; Zhu, Q.; Xu, Y.; Li, Y.; Li, C.; Zhao, W.; Liang, Z.; Chen, L. The efficacy and safety of low-frequency rotating static magnetic field therapy combined with chemotherapy on advanced lung cancer patients: A randomized, double-blinded, controlled clinical trial. Int. J. Radiat. Biol. 2020, 96, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Liu, X.; Shu, Y.S.; Yu, Y.G. Progress of the Impact of Terahertz Radiation on Ion Channel Kinetics in Neuronal Cells. Neurosci. Bull. 2024, 40, 1960–1974. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Chen, X.; Cui, G.; Yin, S.; Chen, L.; Jiang, J.; Hu, Z.; Xie, H.; Zheng, S.; Zhou, L. Nanosecond pulsed electric field inhibits cancer growth followed by alteration in expressions of NF-κB and Wnt/β-catenin signaling molecules. PLoS ONE 2013, 8, e74322. [Google Scholar] [CrossRef]
- Perigo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef]
- Flores, E.R.; Sawyer, W.G. Engineering cancer’s end: An interdisciplinary approach to confront the complexities of cancer. Cancer Cell 2024, 42, 1133–1137. [Google Scholar] [CrossRef]
- Korshoej, A.R.; Saturnino, G.B.; Rasmussen, L.K.; von Oettingen, G.; Sørensen, J.C.; Thielscher, A. Enhancing Predicted Efficacy of Tumor Treating Fields Therapy of Glioblastoma Using Targeted Surgical Craniectomy: A Computer Modeling Study. PLoS ONE 2016, 11, e0164051. [Google Scholar] [CrossRef]
- Tammam, E.; Said, A.M.; Ibrahim, A.A.; Galal, A.I.A. About the Interstitial Microwave Cancer Ablation: Principles, Advantages and Challenges. IEEE Access 2020, 8, 49685–49694. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Mills, G.B. Overcoming implementation challenges of personalized cancer therapy. Nat. Rev. Clin. Oncol. 2012, 9, 542–548. [Google Scholar] [CrossRef]
- Korshoej, A.R.; Hansen, F.L.; Mikic, N.; von Oettingen, G.; Sørensen, J.C.H.; Thielscher, A. Importance of electrode position for the distribution of tumor treating fields (TTFields) in a human brain. Identification of effective layouts through systematic analysis of array positions for multiple tumor locations. PLoS ONE 2018, 13, e0201957. [Google Scholar] [CrossRef]
- Rangan, K.; Briamonte, C.B.; Higgins, M.; Hoffman, C.; Holtzclaw, S.; McHugh, M.; Meyer, A.; Raber, S.; Schmus, C.; Seidl, K.; et al. Neurocognitive side effects of pediatric brain tumors: A continuing challenge. Neuro-Oncology 2024, 26 (Suppl. 4). [Google Scholar] [CrossRef]
- Sun, X.X.; Yu, Q. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacol. Sin. 2015, 36, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Sharma, N.K.; Sharma, A. Editorial: Revisiting the challenges and opportunities in cancer drug resistance. Front. Mol. Biosci. 2024, 11, 1497754. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.M.; Tian, X.F.; Feng, S.; Zhang, L.; Wang, J.J.; Guo, R.W.; Zhu, Y.M.; Yu, X.; Zhang, Y.S.; Du, H.F.; et al. Intermittent F-actin Perturbations by Magnetic Fields Inhibit Breast Cancer Metastasis. Research 2023, 6, 0080. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Q.J.; Zhu, Q.Y.; Yang, X.J.; Li, Z.B.; Fu, J. Applications of machine learning in tumor-associated macrophages. Front. Immunol. 2022, 13, 985863. [Google Scholar] [CrossRef]
- Besler, E.; Wang, Y.C.; Sahakian, A.V. Real-Time Radiofrequency Ablation Lesion Depth Estimation Using Multi-frequency Impedance With a Deep Neural Network and Tree-Based Ensembles. IEEE Trans. Biomed. Eng. 2020, 67, 1890–1899. [Google Scholar] [CrossRef]
- Briz, P.; López-Alonso, B.; Sarnago, H.; Burdío, J.M.; Lucía, O. Tumor location on electroporation therapies by means of multi-electrode structures and machine learning. Bioelectrochemistry 2023, 154, 108510. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, Y.Y.; Wu, Q.; You, Y.; Lan, Z.; Zou, K.L.; Cheng, G.W.; Chen, H.; Han, Y.H.; Chen, Y.; et al. Microwave-responsive gadolinium metal-organic frameworks nanosystem for MRI-guided cancer thermotherapy and synergistic immunotherapy. Bioact. Mater. 2024, 33, 532–544. [Google Scholar] [CrossRef]
- Giladi, M.; Voloshin, T.; Shteingauz, A.; Munster, M.; Blat, R.; Porat, Y.; Schneiderman, R.S.; Cahal, S.; Itzhaki, A.; Kirson, E.; et al. The antitumor activity of alternating electric fields (TTFields) in combination with immune checkpoint inhibitors. J. Clin. Oncol. 2016, 34, e14570. [Google Scholar] [CrossRef]
- Diamant, G.; Goldman, H.S.; Plotnitsky, L.G.; Roitman, M.; Shiloach, T.; Globerson-Levin, A.; Eshhar, Z.; Haim, O.; Pencovich, N.; Grossman, R.; et al. T Cells Retain Pivotal Antitumoral Functions under Tumor-Treating Electric Fields. J. Immunol. 2021, 207, 709–719. [Google Scholar] [CrossRef]
- Barsheshet, Y.; Voloshin, T.; Brant, B.; Cohen, G.; Avigdor, L.; Blatt, R.; Cahal, S.; Khalil, T.H.; Zemer-Tov, E.; Paz, R.; et al. 860 In vivo effectiveness of tumor treating fields (TTFields) concomitant with immune checkpoint inhibitors in non-small cell lung cancer (NSCLC). J. Immunother. Cancer 2022, 10, A899. [Google Scholar] [CrossRef]
- Prasad, R.; Jain, N.K.; Conde, J.; Srivastava, R. Localized nanotheranostics: Recent developments in cancer nanomedicine. Mater. Today Adv. 2020, 8, 100087. [Google Scholar] [CrossRef]
- Luiz, M.T.; Dutra, J.A.P.; Viegas, J.S.R.; de Araujo, J.T.C.; Tavares, A.G., Jr.; Chorilli, M. Hybrid Magnetic Lipid-Based Nanoparticles for Cancer Therapy. Pharmaceutics 2023, 15, 751. [Google Scholar] [CrossRef]
- Singh, B.; Ma, S.L.; Hara, T.O.; Singh, S. Nanomaterials-Based Biosensors for the Detection of Prostate Cancer Biomarkers: Recent Trends and Future Perspective. Adv. Mater. Technol. 2023, 8, 2201860. [Google Scholar] [CrossRef]
- Kim, Y.; Chae, J.K.; Lee, J.H.; Choi, E.; Lee, Y.K.; Song, J. Free manipulation system for nanorobot cluster based on complicated multi-coil electromagnetic actuator. Sci. Rep. 2021, 11, 19756. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.C.; del Mar, S.A.M.; Irene, C.; Sandra, R.A.; Josefa, L.; Elisa, R.M.; Nicolás, O.; Isabel, N.M. Could Radiotherapy Effectiveness Be Enhanced by Electromagnetic Field Treatment? Int. J. Mol. Sci. 2013, 14, 14974–14995. [Google Scholar] [CrossRef] [PubMed]
- Dono, A.; Mitra, S.; Shah, M.; Takayasu, T.; Zhu, J.J.; Tandon, N.; Patel, C.B.; Esquenazi, Y.; Ballester, L.Y. PTEN mutations predict benefit from tumor treating fields (TTFields) therapy in patients with recurrent glioblastoma. J. Neuro-Oncol. 2021, 153, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Pohling, C.; Nguyen, H.; Chang, E.; Schubert, K.E.; Nie, Y.; Bashkirov, V.; Yamamoto, V.; Zeng, Y.; Stupp, R.; Schulte, R.W.; et al. Current status of the preclinical evaluation of alternating electric fields as a form of cancer therapy. Bioelectrochemistry 2023, 149, 108287. [Google Scholar] [CrossRef]
- Sengupta, S.; Balla, V.K. A review on the use of magnetic fields and ultrasound for non-invasive cancer treatment. J. Adv. Res. 2018, 14, 97–111. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Peng, X.; Xu, T.; Lin, Z.; Sun, M.; Li, Y.; Xian, Q.; Xiao, T.; Chen, S.; Xie, Y.; et al. Precise Electromagnetic Modulation of the Cell Cycle and Its Applications in Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 4445. https://doi.org/10.3390/ijms26094445
Shi K, Peng X, Xu T, Lin Z, Sun M, Li Y, Xian Q, Xiao T, Chen S, Xie Y, et al. Precise Electromagnetic Modulation of the Cell Cycle and Its Applications in Cancer Therapy. International Journal of Molecular Sciences. 2025; 26(9):4445. https://doi.org/10.3390/ijms26094445
Chicago/Turabian StyleShi, Keni, Xiqing Peng, Ting Xu, Ziqi Lin, Mingyu Sun, Yiran Li, Qingyi Xian, Tingting Xiao, Siyuan Chen, Ying Xie, and et al. 2025. "Precise Electromagnetic Modulation of the Cell Cycle and Its Applications in Cancer Therapy" International Journal of Molecular Sciences 26, no. 9: 4445. https://doi.org/10.3390/ijms26094445
APA StyleShi, K., Peng, X., Xu, T., Lin, Z., Sun, M., Li, Y., Xian, Q., Xiao, T., Chen, S., Xie, Y., Zhang, R., Zeng, J., & Xu, B. (2025). Precise Electromagnetic Modulation of the Cell Cycle and Its Applications in Cancer Therapy. International Journal of Molecular Sciences, 26(9), 4445. https://doi.org/10.3390/ijms26094445





