Maternal Serum 25-Hydroxyvitamin D as a Possible Modulator of Fetal Adiposity: A Prospective Longitudinal Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| EFA | Estimated fetal adiposity |
| BMI | Body mass index |
| GDM | Gestational diabetes mellitus |
| 25(OH)D | 25-hydroxyvitamin D |
References
- ACOG Practice Bulletin No. 204: Fetal Growth Restriction. Obstet. Gynecol. 2019, 133, e97–e109. [CrossRef]
- Macrosomia: ACOG Practice Bulletin, Number 216. Obstet. Gynecol. 2020, 135, e18–e35. [CrossRef] [PubMed]
- Yu, Z.B.; Han, S.P.; Zhu, G.Z.; Zhu, C.; Wang, X.J.; Cao, X.G.; Guo, X.R. Birth weight and subsequent risk of obesity: A systematic review and meta-analysis. Obes. Rev. 2011, 12, 525–542. [Google Scholar] [CrossRef]
- Waters, E.; de Silva-Sanigorski, A.; Hall, B.J.; Brown, T.; Campbell, K.J.; Gao, Y.; Armstrong, R.; Prosser, L.; Summerbell, C.D. Interventions for preventing obesity in children. Cochrane Database Syst. Rev. 2011, 12, Cd001871. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Alfirevic, Z.; Da Silva Costa, F.; Deter, R.L.; Figueras, F.; Ghi, T.; Glanc, P.; Khalil, A.; Lee, W.; Napolitano, R.; et al. ISUOG Practice Guidelines: Ultrasound assessment of fetal biometry and growth. Ultrasound Obstet. Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.W. Human intrauterine growth and nutrient accretion. Semin. Perinatol. 1984, 8, 74–93. [Google Scholar]
- Ikenoue, S.; Waffarn, F.; Sumiyoshi, K.; Ohashi, M.; Ikenoue, C.; Buss, C.; Gillen, D.L.; Simhan, H.N.; Entringer, S.; Wadhwa, P.D. Association of ultrasound-based measures of fetal body composition with newborn adiposity. Pediatr. Obes. 2017, 12 (Suppl. S1), 86–93. [Google Scholar] [CrossRef]
- Ikenoue, S.; Waffarn, F.; Sumiyoshi, K.; Ohashi, M.; Ikenoue, C.; Tanaka, M.; Gillen, D.L.; Buss, C.; Entringer, S.; Wadhwa, P.D. Maternal insulin resistance in pregnancy is associated with fetal fat deposition: Findings from a longitudinal study. Am. J. Obstet. Gynecol. 2023, 228, 455.e1–455.e8. [Google Scholar] [CrossRef]
- Crowe, F.L.; Mughal, M.Z.; Maroof, Z.; Berry, J.; Kaleem, M.; Abburu, S.; Walraven, G.; Masher, M.I.; Chandramohan, D.; Manaseki-Holland, S. Vitamin D for Growth and Rickets in Stunted Children: A Randomized Trial. Pediatrics 2021, 147, e20200815. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Wang, Y.; Zhao, J.; Li, H.; Shen, Q.; Wang, X.; Ni, M.; Ouyang, F.; Vinturache, A.; et al. Relationship of maternal obesity and vitamin D concentrations with fetal growth in early pregnancy. Eur. J. Nutr. 2022, 61, 915–924. [Google Scholar] [CrossRef]
- Plesner, J.L.; Dahl, M.; Fonvig, C.E.; Nielsen, T.R.H.; Kloppenborg, J.T.; Pedersen, O.; Hansen, T.; Holm, J.C. Obesity is associated with vitamin D deficiency in Danish children and adolescents. J. Pediatr. Endocrinol. Metab. 2018, 31, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-Diamond, D.; Baylin, A.; Mora-Plazas, M.; Marin, C.; Arsenault, J.E.; Hughes, M.D.; Willett, W.C.; Villamor, E. Vitamin D deficiency and anthropometric indicators of adiposity in school-age children: A prospective study. Am. J. Clin. Nutr. 2010, 92, 1446–1451. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal relationship between obesity and vitamin D status: Bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef]
- Bahadorpour, S.; Hajhashemy, Z.; Saneei, P. Serum 25-hydroxyvitamin D levels and dyslipidemia: A systematic review and dose-response meta-analysis of epidemiologic studies. Nutr. Rev. 2022, 81, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Silvagno, F.; Pescarmona, G. Spotlight on vitamin D receptor, lipid metabolism and mitochondria: Some preliminary emerging issues. Mol. Cell. Endocrinol. 2017, 450, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Christesen, H.T.; Elvander, C.; Lamont, R.F.; Jørgensen, J.S. The impact of vitamin D in pregnancy on extraskeletal health in children: A systematic review. Acta Obstet. Gynecol. Scand. 2012, 91, 1368–1380. [Google Scholar] [CrossRef]
- Andersen, L.B.; Abrahamsen, B.; Dalgård, C.; Kyhl, H.B.; Beck-Nielsen, S.S.; Frost-Nielsen, M.; Jørgensen, J.S.; Barington, T.; Christesen, H.T. Parity and tanned white skin as novel predictors of vitamin D status in early pregnancy: A population-based cohort study. Clin. Endocrinol. 2013, 79, 333–341. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.; Hu, Y.; Wang, Y.; Wu, Y.; Lian, F.; Li, H.; Yang, J.; Xu, X. The effects of vitamin D supplementation on glycemic control and maternal-neonatal outcomes in women with established gestational diabetes mellitus: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 3148–3157. [Google Scholar] [CrossRef]
- Heyden, E.L.; Wimalawansa, S.J. Vitamin D: Effects on human reproduction, pregnancy, and fetal well-being. J. Steroid Biochem. Mol. Biol. 2018, 180, 41–50. [Google Scholar] [CrossRef]
- Crozier, S.R.; Harvey, N.C.; Inskip, H.M.; Godfrey, K.M.; Cooper, C.; Robinson, S.M. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: Findings from the Southampton Women’s Survey. Am. J. Clin. Nutr. 2012, 96, 57–63. [Google Scholar] [CrossRef]
- Morales, E.; Rodriguez, A.; Valvi, D.; Iñiguez, C.; Esplugues, A.; Vioque, J.; Marina, L.S.; Jiménez, A.; Espada, M.; Dehli, C.R.; et al. Deficit of vitamin D in pregnancy and growth and overweight in the offspring. Int. J. Obes. 2015, 39, 61–68. [Google Scholar] [CrossRef]
- Daraki, V.; Roumeliotaki, T.; Chalkiadaki, G.; Katrinaki, M.; Karachaliou, M.; Leventakou, V.; Vafeiadi, M.; Sarri, K.; Vassilaki, M.; Papavasiliou, S.; et al. Low maternal vitamin D status in pregnancy increases the risk of childhood obesity. Pediatr. Obes. 2018, 13, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, L.; Li, P. Vitamin D in gestational diabetes: A broadened frontier. Clin. Chim. Acta 2022, 537, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Akiba, Y.; Ikenoue, S.; Endo, T.; Kasuga, Y.; Ochiai, D.; Miyakoshi, K.; Ishii, R.; Yakubo, K.; Tanaka, M. Differences in fetal fractional limb volume changes in normal and gestational diabetic pregnancies: An exploratory observational study. BJOG 2021, 128, 329–335. [Google Scholar] [CrossRef]
- Miliku, K.; Felix, J.F.; Voortman, T.; Tiemeier, H.; Eyles, D.W.; Burne, T.H.; McGrath, J.J.; Jaddoe, V.W.V. Associations of maternal and fetal vitamin D status with childhood body composition and cardiovascular risk factors. Matern. Child. Nutr. 2019, 15, e12672. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.D.; Christensen, M.E.; Dalgård, C.; Lykkedegn, S.; Andersen, L.B.; Andersen, M.S.; Glintborg, D.; Christesen, H.T. Pregnancy or cord 25-hydroxyvitamin D is not associated with measures of body fat or adiposity in children from three months to three years of age. An Odense Child Cohort study. Clin. Nutr. 2020, 39, 1832–1839. [Google Scholar] [CrossRef]
- Jackson, J.L.; Judd, S.E.; Panwar, B.; Howard, V.J.; Wadley, V.G.; Jenny, N.S.; Gutiérrez, O.M. Associations of 25-hydroxyvitamin D with markers of inflammation, insulin resistance and obesity in black and white community-dwelling adults. J. Clin. Transl. Endocrinol. 2016, 5, 21–25. [Google Scholar] [CrossRef][Green Version]
- Bi, W.G.; Nuyt, A.M.; Weiler, H.; Leduc, L.; Santamaria, C.; Wei, S.Q. Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta-analysis. JAMA Pediatr. 2018, 172, 635–645. [Google Scholar] [CrossRef]
- Krishnaveni, G.V.; Veena, S.R.; Winder, N.R.; Hill, J.C.; Noonan, K.; Boucher, B.J.; Karat, S.C.; Fall, C.H. Maternal vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: The Mysore Parthenon Study. Am. J. Clin. Nutr. 2011, 93, 628–635. [Google Scholar] [CrossRef]
- Asano, L.; Watanabe, M.; Ryoden, Y.; Usuda, K.; Yamaguchi, T.; Khambu, B.; Takashima, M.; Sato, S.I.; Sakai, J.; Nagasawa, K.; et al. Vitamin D Metabolite, 25-Hydroxyvitamin D, Regulates Lipid Metabolism by Inducing Degradation of SREBP/SCAP. Cell Chem. Biol. 2017, 24, 207–217. [Google Scholar] [CrossRef]
- Cho, H.J.; Kang, H.C.; Choi, S.A.; Ju, Y.C.; Lee, H.S.; Park, H.J. The possible role of Ca2+ on the activation of microsomal triglyceride transfer protein in rat hepatocytes. Biol. Pharm. Bull. 2005, 28, 1418–1423. [Google Scholar] [CrossRef]
- Zemel, M.B.; Shi, H.; Greer, B.; Dirienzo, D.; Zemel, P.C. Regulation of adiposity by dietary calcium. Faseb J. 2000, 14, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; She, G.T.; Sun, L.Z.; Lu, H.; Wang, Y.P.; Miao, J.; Liu, K.Z.; Sun, C.F.; Ju, H.H. Correlation of serum vitamin D, adipose tissue vitamin D receptor, and peroxisome proliferator-activated receptor γ in women with gestational diabetes mellitus. Chin. Med. J. 2019, 132, 2612–2620. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.; Lim, Y. Vitamin D3 improves lipophagy-associated renal lipid metabolism and tissue damage in diabetic mice. Nutr. Res. 2020, 80, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.A.; Ashraf, A. Role of vitamin d in insulin secretion and insulin sensitivity for glucose homeostasis. Int. J. Endocrinol. 2010, 2010, 351385. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.K.B.; Sulis, P.M.; Cavalari, F.C.; Padilla, D.P.R.; Aragón, M.; Gaspar, J.M.; Silva, F. 1α,25-(OH)2 vitamin D3 prevents insulin resistance and regulates coordinated exocytosis and insulin secretion. J. Nutr. Biochem. 2022, 99, 108864. [Google Scholar] [CrossRef]
- Kjalarsdottir, L.; Tersey, S.A.; Vishwanath, M.; Chuang, J.C.; Posner, B.A.; Mirmira, R.G.; Repa, J.J. 1,25-Dihydroxyvitamin D3 enhances glucose-stimulated insulin secretion in mouse and human islets: A role for transcriptional regulation of voltage-gated calcium channels by the vitamin D receptor. J. Steroid Biochem. Mol. Biol. 2019, 185, 17–26. [Google Scholar] [CrossRef]
- Ji, J.L.; Muyayalo, K.P.; Zhang, Y.H.; Hu, X.H.; Liao, A.H. Immunological function of vitamin D during human pregnancy. Am. J. Reprod. Immunol. 2017, 78, e12716. [Google Scholar] [CrossRef]
- Griffith, R.J.; Alsweiler, J.; Moore, A.E.; Brown, S.; Middleton, P.; Shepherd, E.; Crowther, C.A. Interventions to prevent women from developing gestational diabetes mellitus: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2020, 6, CD012394. [Google Scholar] [CrossRef]
- Dabrowski, F.A.; Grzechocinska, B.; Wielgos, M. The role of vitamin D in reproductive health—A Trojan Horse or the Golden Fleece? Nutrients 2015, 7, 4139–4153. [Google Scholar] [CrossRef]
- Tirabassi, G.; Cutini, M.; Muscogiuri, G.; Delli Muti, N.; Corona, G.; Galdiero, M.; Pivonello, R.; Colao, A.; Balercia, G. Association between vitamin D and sperm parameters: Clinical evidence. Endocrine 2017, 58, 194–198. [Google Scholar] [CrossRef]
- Makker, K.; Zhang, M.; Wang, G.; Hong, X.; Aziz, K.B.; Wang, X. Maternal and fetal factors affecting cord plasma leptin and adiponectin levels and their ratio in preterm and term newborns: New insight on fetal origins of metabolic dysfunction. Precis. Nutr. 2022, 1, e00013. [Google Scholar] [PubMed]
- Rabinowich, A.; Avisdris, N.; Yehuda, B.; Vanetik, S.; Khawaja, J.; Graziani, T.; Neeman, B.; Wexler, Y.; Specktor-Fadida, B.; Herzlich, J.; et al. Fetal body composition reference charts and sexual dimorphism using magnetic resonance imaging. Am. J. Clin. Nutr. 2024, 120, 1364–1372. [Google Scholar] [CrossRef]
- Zapata-Masias, Y.; Marqueta, B.; Gómez Roig, M.D.; Gonzalez-Bosquet, E. Obstetric and perinatal outcomes in women ≥ 40 years of age: Associations with fetal growth disorders. Early Hum. Dev. 2016, 100, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Japan Society of Ultrasound in Medicine. Ultrasound Fetal measurement standardization and Japanese standard. J. Med. Ultrasonics 2003, 30, J416–J440. [Google Scholar]
- Itakura, A.; Shoji, S.; Shigeru, A.; Kotaro, F.; Junichi, H.; Hironobu, H.; Kamei, Y.; Eiji, K.; Shintaro, M.; Ryu, M.; et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology and Japan Association of Obstetricians and Gynecologists 2020 edition. J. Obstet. Gynaecol. Res. 2023, 49, 5–53. [Google Scholar] [CrossRef]
- Itabashi, K.; Miura, F.; Uehara, R.; Nakamura, Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr. Int. 2014, 56, 702–708. [Google Scholar] [CrossRef]

| Characteristics | Mean ± SD or N (%) |
|---|---|
| Age, years | 34.9 ± 3.9 |
| Pre-pregnancy BMI, kg/m2 | 20.9 ± 2.7 |
| Primiparous | 55 (62%) |
| Gestational weight gain, kg | 10.5 ± 3.6 |
| Gestational diabetes | 21 (24%) |
| Hypertensive disorders in pregnancy | 6 (6.7%) |
| Birth weight, g | 3026 ± 330 |
| Birth weight percentile, % | 58.9 ± 26.5 |
| Gestational age at birth, week | 38.9 ± 1.0 |
| Infant sex (female) | 43 (48%) |
| Maternal serum 25-hydroxyvitamin D, ng/mL | |
| at 10 weeks | 18.8 ± 8.2 |
| at 24 weeks | 16.5 ± 8.3 |
| at 30 weeks | 18.0 ± 9.2 |
| at 36 weeks | 16.4 ± 9.2 |
| Parameters | 24 Weeks | 30 Weeks | 36 Weeks |
|---|---|---|---|
| Estimated fetal weight, g | 764 ± 153 | 1572 ± 153 | 2562 ± 247 |
| Arm total area, mm2 | 2.84 ± 0.56 | 4.69 ± 0.77 | 7.97 ± 1.21 |
| Arm lean area, mm2 | 1.50 ± 0.31 | 2.30 ± 0.39 | 3.47 ± 0.55 |
| Arm fat area, mm2 | 1.33 ± 0.40 | 2.40 ± 0.56 | 4.50 ± 0.94 |
| Thigh total area, mm2 | 5.73 ± 1.09 | 10.40 ± 1.51 | 17.36 ± 2.75 |
| Thigh lean area, mm2 | 3.56 ± 0.64 | 6.04 ± 0.96 | 9.38 ± 1.48 |
| Thigh fat area, mm2 | 2.17 ± 0.67 | 4.36 ± 0.93 | 7.98 ± 1.78 |
| Anterior abdominal wall thickness, mm | 2.25 ± 0.53 | 2.92 ± 0.61 | 4.04 ± 0.96 |
| EFA | 0.01 ± 0.64 | 0.03 ± 0.66 | 0.00 ± 0.64 |
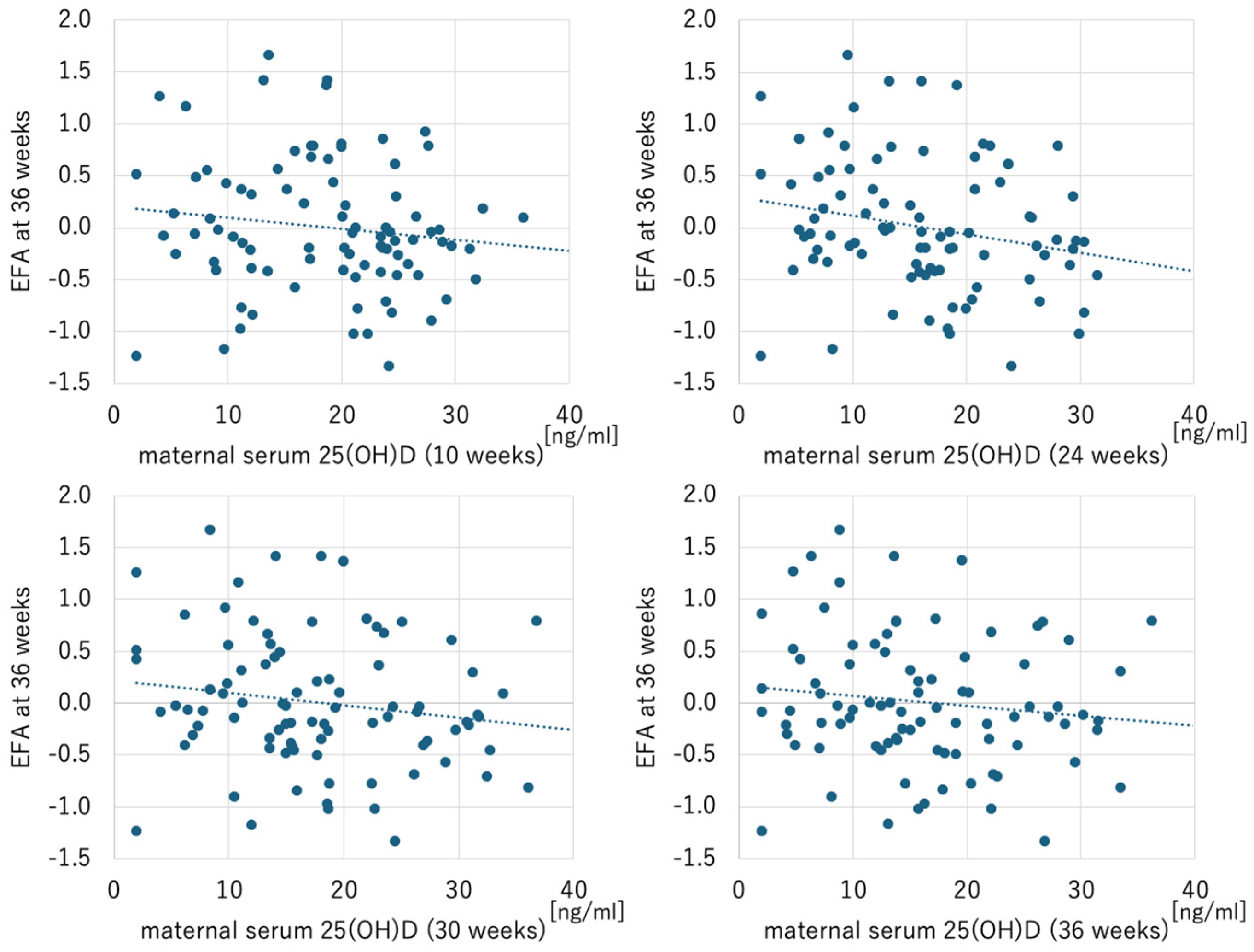
| Parameters | EFA at 24 Weeks | EFA at 30 Weeks | EFA at 36 Weeks | |||
|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | |
| Maternal serum 25(OH)D | ||||||
| at 10 weeks | −0.209 | 0.051 | −0.047 | 0.661 | −0.136 | 0.205 |
| at 24 weeks | −0.019 | 0.861 | −0.101 | 0.347 | −0.234 | 0.028 |
| at 30 weeks | −0.034 | 0.754 | −0.138 | 0.197 | −0.174 | 0.103 |
| at 36 weeks | −0.063 | 0.557 | −0.083 | 0.442 | −0.137 | 0.200 |
| Parameters | Total (n = 89) | GDM (n = 21) | Non-GDM (n = 68) | |||
|---|---|---|---|---|---|---|
| Unstandardized B [95%CI] | p-Value | Unstandardized B [95%CI] | p-Value | Unstandardized B [95%CI] | p-Value | |
| Maternal serum 25(OH)D | ||||||
| at 10 weeks | −0.007 [−0.024–0.009] | 0.391 | −0.049 [−0.088–−0.010] | 0.017 | −0.002 [−0.020–0.017] | 0.864 |
| at 24 weeks | −0.012 [−0.028–0.004] | 0.134 | −0.042 [−0.071–−0.013] | 0.008 | −0.005 [−0.023–0.014] | 0.619 |
| at 30 weeks | −0.007 [−0.021–0.008] | 0.378 | −0.045 [−0.080–−0.010] | 0.014 | 0.000 [−0.017–0.016] | 0.971 |
| at 36 weeks | −0.005 [−0.021–0.090] | 0.461 | −0.044 [−0.093–0.006] | 0.089 | −0.002 [−0.018–0.013] | 0.766 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akita, K.; Ikenoue, S.; Tamai, J.; Otani, T.; Fukutake, M.; Kasuga, Y.; Tanaka, M. Maternal Serum 25-Hydroxyvitamin D as a Possible Modulator of Fetal Adiposity: A Prospective Longitudinal Study. Int. J. Mol. Sci. 2025, 26, 4435. https://doi.org/10.3390/ijms26094435
Akita K, Ikenoue S, Tamai J, Otani T, Fukutake M, Kasuga Y, Tanaka M. Maternal Serum 25-Hydroxyvitamin D as a Possible Modulator of Fetal Adiposity: A Prospective Longitudinal Study. International Journal of Molecular Sciences. 2025; 26(9):4435. https://doi.org/10.3390/ijms26094435
Chicago/Turabian StyleAkita, Keisuke, Satoru Ikenoue, Junko Tamai, Toshimitsu Otani, Marie Fukutake, Yoshifumi Kasuga, and Mamoru Tanaka. 2025. "Maternal Serum 25-Hydroxyvitamin D as a Possible Modulator of Fetal Adiposity: A Prospective Longitudinal Study" International Journal of Molecular Sciences 26, no. 9: 4435. https://doi.org/10.3390/ijms26094435
APA StyleAkita, K., Ikenoue, S., Tamai, J., Otani, T., Fukutake, M., Kasuga, Y., & Tanaka, M. (2025). Maternal Serum 25-Hydroxyvitamin D as a Possible Modulator of Fetal Adiposity: A Prospective Longitudinal Study. International Journal of Molecular Sciences, 26(9), 4435. https://doi.org/10.3390/ijms26094435






