The Influence of the Non-Pathogenic Fusarium oxysporum Fo47 Strain on Flax Resistance to Pathogens
Abstract
1. Introduction
2. Results and Discussion
2.1. Antagonistic Effect of Non-Pathogenic Strain F. oxysporum Fo47 on Pathogenic F. oxysporum
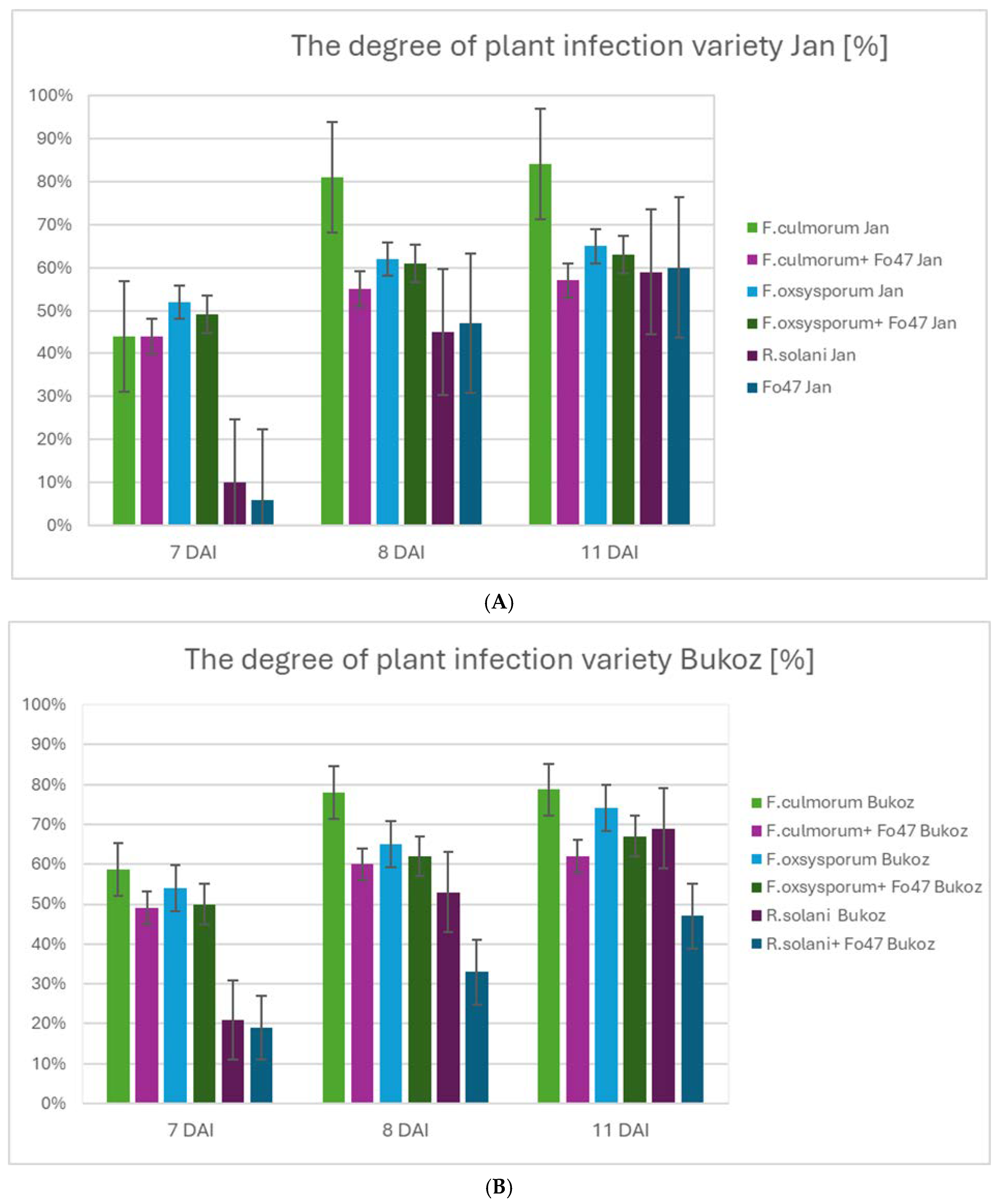
2.2. Determination of Flax Resistance to Fungi and Impact of Non-Pathogenic Strain Fo47
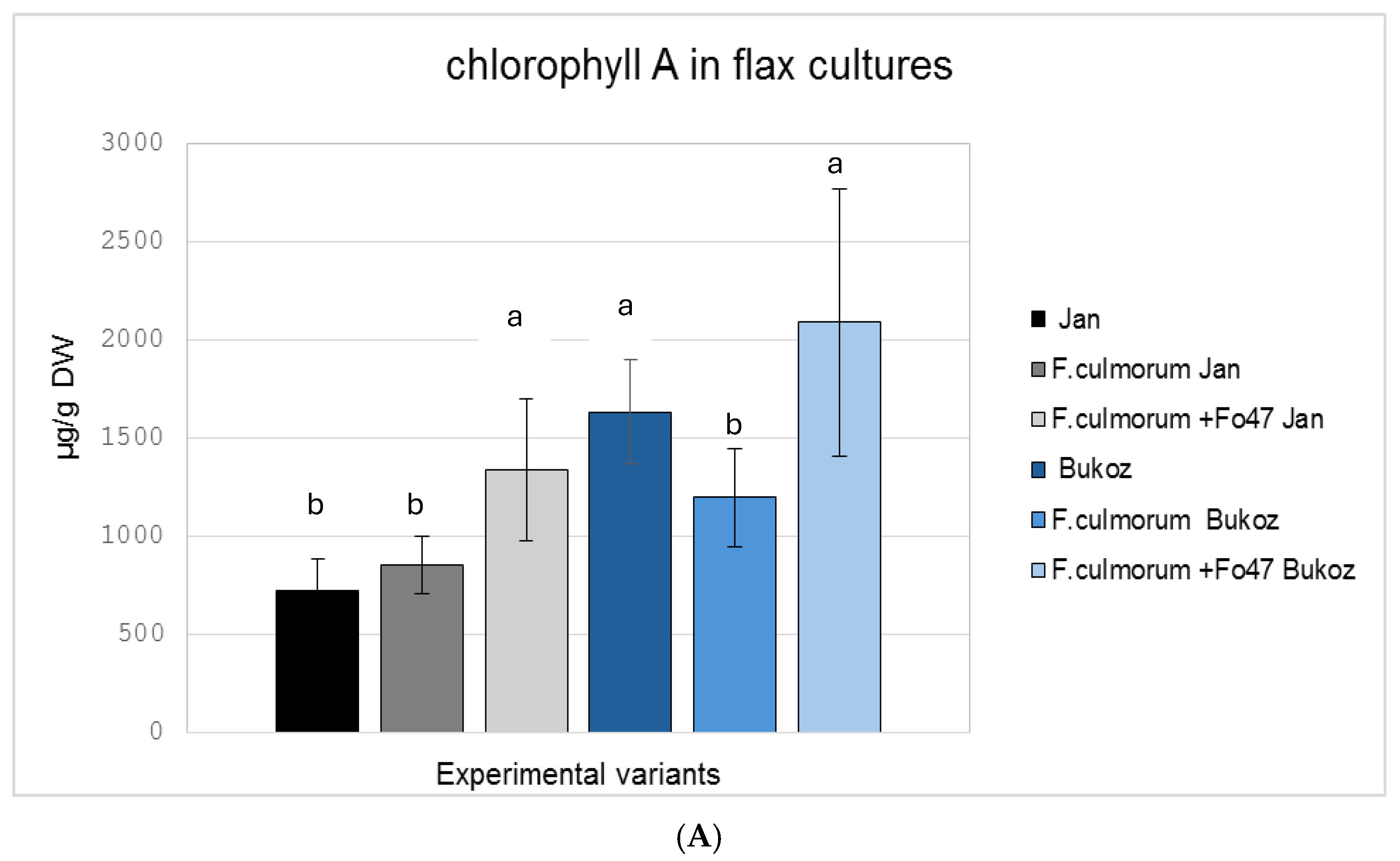
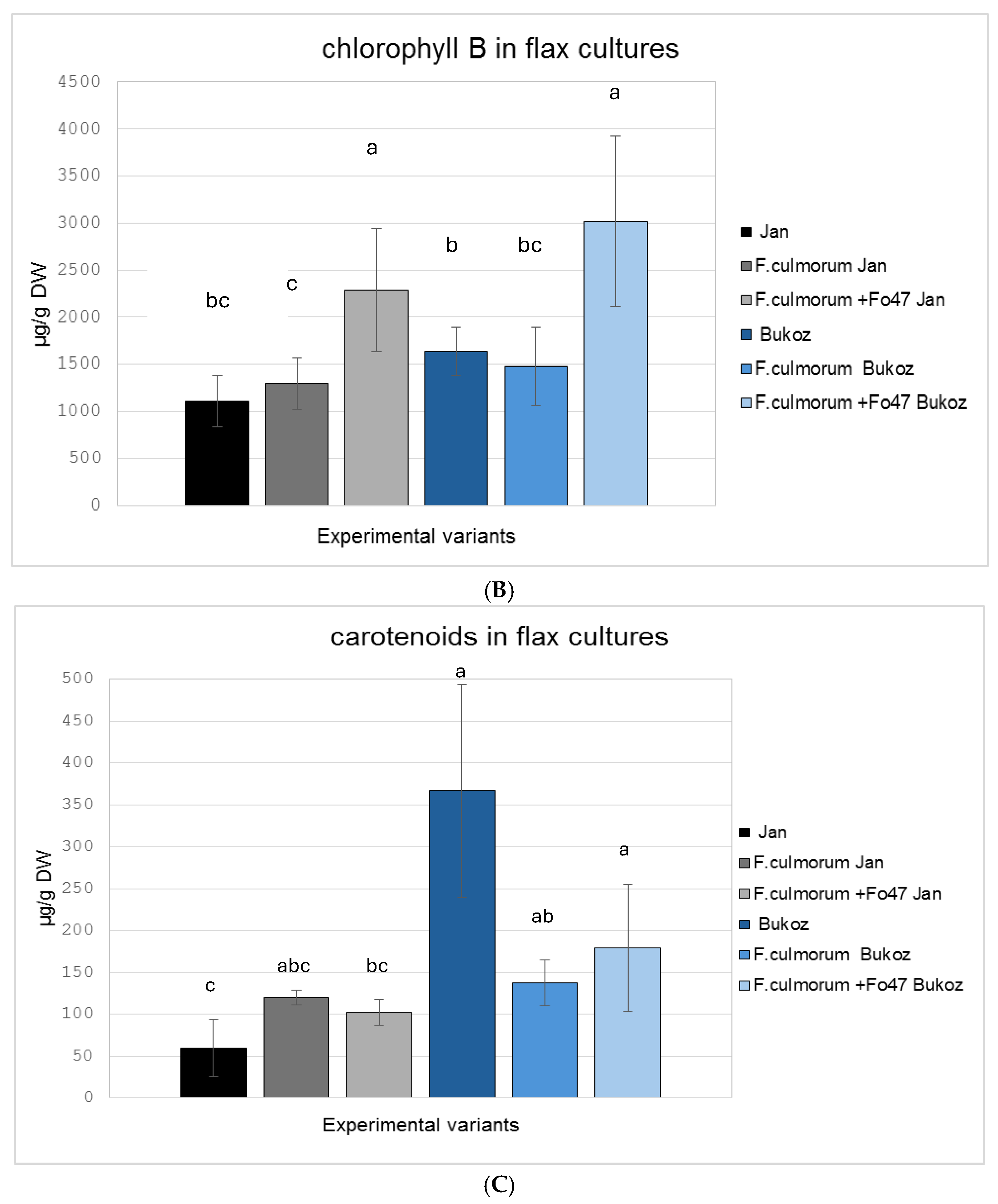
2.3. Determination of the Pigment Content in Flax in In Vitro Cultures
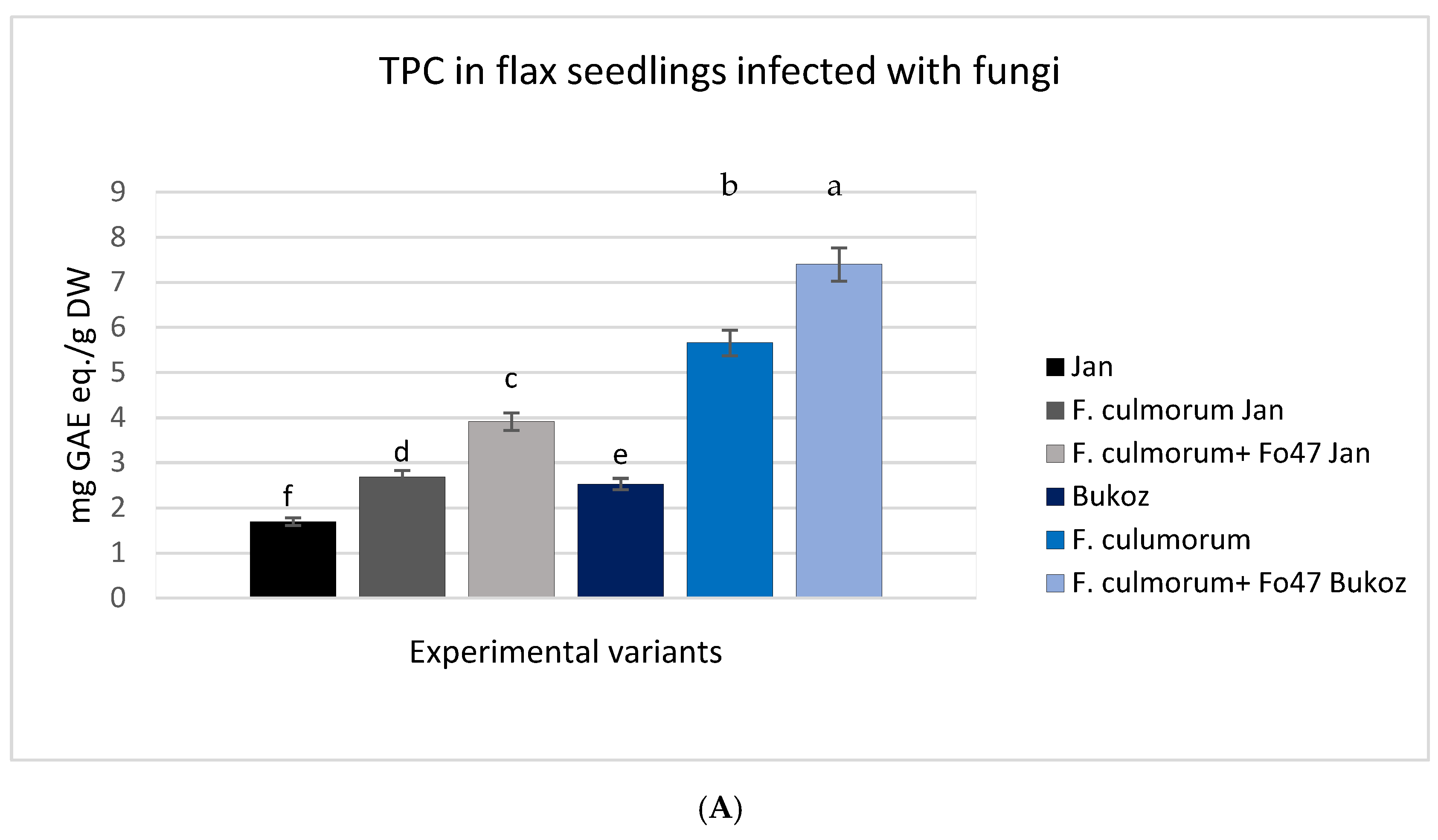
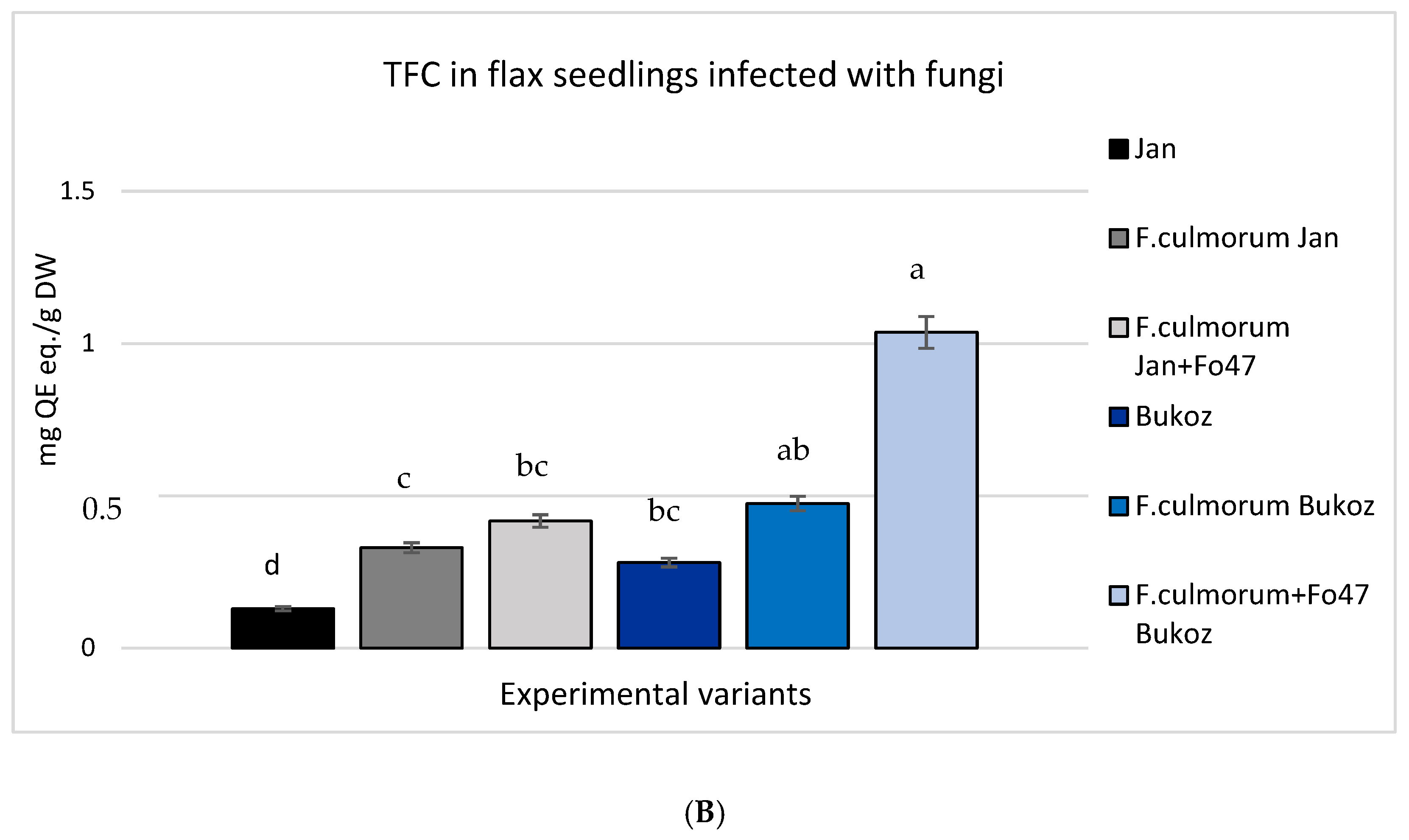
2.4. Analysis of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) in Tested Flax Plants
2.5. Spectroscopic Analysis of Cell Wall Polymers in Studied Flax Plants
3. Materials and Methods
3.1. Plant Material
3.2. Fungal Material
3.3. Conditions of Fungal Cultures
3.4. Determination of Biocontrol Activity of Fo47 Against F. oxysporum
3.5. Flax–Fungi Bioassay Determining the Resistance of Tested Flax Plants to Fungal Diseases
3.6. Analysis of Content of Photosynthetically Active Pigments in Tested Flax Plants
3.7. Determination of Total Polyphenol Content (TPC) and Total Flavonoid Content (TFC)
3.8. Analysis of FTIR Spectra
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jhala, A.J.; Hall, L.M. Flax (Linum usitatissimum L.): Current uses and future applications. Aust. J. Basic Appl. Sci. 2010, 4, 4304–4312. [Google Scholar]
- Stavropoulos, P.; Mavroeidis, A.; Papadopoulos, G.; Roussis, I.; Bilalis, D.; Kakabouki, I. On the path towards a “Greener” EU: A mini review on flax (Linum usitatissimum L.) as a case study. Plants 2023, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Mueed, A.; Ali, A.; Madjirebaye, P.; Li, J.; Deng, Z. Metabolomic and chemometrics depict to understand the nutraceutical potential of different flaxseed varieties. Food Biosci. 2024, 59, 103876. [Google Scholar] [CrossRef]
- Czemplik, M.; Boba, A.; Kostyn, K.; Kulma, A.; Mituła, A.; Sztajnert, M.; Skórkowska-Telichowska, K. Flax engineering for biomedical application. In Biomedical Engineering: Trends, Research and Technologies; IntechOpen: London, UK, 2011; Volume 17. [Google Scholar]
- Moyse, J.; Lecomte, S.; Marcou, S.; Mongelard, G.; Gutierrez, L.; Höfte, M. Overview and management of the most common eukaryotic diseases of flax (Linum usitatissimum). Plants 2023, 12, 2811. [Google Scholar] [CrossRef]
- Dmitriev, A.A.; Krasnov, G.S.; Rozhmina, T.A.; Novakovskiy, R.O.; Snezhkina, A.V.; Fedorova, M.S.; Yurkevich, O.Y.; Muravenko, O.V.; Bolsheva, N.L.; Kudryavtseva, A.V.; et al. Differential Gene Expression in Response to Fusarium oxysporum Infection in Resistant and Susceptible Genotypes of Flax (Linum usitatissimum L.). BMC Plant Biol. 2017, 17, 253. [Google Scholar] [CrossRef]
- Alabouvette, C. Fusarium-wilt suppressive soils from the Châteaurenard region: Review of a 10-year study. Agronomie 1986, 6, 273–284. [Google Scholar] [CrossRef]
- Aimé, S.; Alabouvette, C.; Steinberg, C.; Olivain, C. The endophytic strain Fusarium oxysporum Fo47: A good candidate for priming the defense responses in tomato roots. Mol. Plant-Microbe Interact. 2013, 26, 918–926. [Google Scholar] [CrossRef]
- Constantin, M.E.; De Lamo, F.J.; Vlieger, B.V.; Rep, M.; Takken, F.L. Endophyte-mediated resistance in tomato to Fusarium oxysporum is independent of ET, JA, and SA. Front. Plant Sci. 2019, 10, 979. [Google Scholar] [CrossRef]
- Veloso, J.F.C.A.; Díaz, J. Fusarium oxysporum Fo47 confers protection to pepper plants against Verticillium dahliae and Phytophthora capsici, and induces the expression of defence genes. Plant Pathol. 2012, 61, 281–288. [Google Scholar] [CrossRef]
- Veloso, J.; Alabouvette, C.; Olivain, C.; Flors, V.; Pastor, V.; García, T.; Díaz, J. Modes of action of the protective strain Fo47 in controlling Verticillium wilt of pepper. Plant Pathol. 2016, 65, 997–1007. [Google Scholar] [CrossRef]
- Benhamou, N.; Garand, C. Cytological analysis of defense-related mechanisms induced in pea root tissues in response to colonization by nonpathogenic Fusarium oxysporum Fo47. Phytopathology 2001, 91, 730–740. [Google Scholar] [CrossRef]
- Constantin, M.E.; de Lamo, F.J.; Rep, M.; Takken, F.L. From laboratory to field: Applying the Fo47 biocontrol strain in potato fields. Eur. J. Plant Pathol. 2020, 158, 645–654. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, J.; Singh, R.S. Nonpathogenic Fusarium as a biological control agent. Plant Pathol. J. 2011, 9, 79–91. [Google Scholar] [CrossRef]
- Alabouvette, C.; Olivain, C.; Migheli, Q.; Steinberg, C. Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. New Phytol. 2009, 184, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Le Floch, G.; Rey, P.; Benhamou, N.; Tirilly, Y. Combining the oomycete Pythium oligandrum with two other antagonistic fungi: Root relationships and tomato grey mold biocontrol. Biol. Control 2009, 50, 288–298. [Google Scholar] [CrossRef]
- Wróbel-Kwiatkowska, M.; Osika, A.; Liszka, J.; Lipiński, M.; Dymińska, L.; Piegza, M.; Rymowicz, W. The impact of a non-pathogenic strain of Fusarium oxysporum on structural and biochemical properties of flax suspension cultures. Int. J. Mol. Sci. 2024, 25, 9616. [Google Scholar] [CrossRef] [PubMed]
- Heller, K.; Liu, L.; Feng, Z.; Ameline, A.; Święcicki, W.; Kazakova, A.; Kazakov, A.; Jankauskiene, Z.; Sawinska, Z. A Comparative Study Between Europe and China in Crop Management of Two Types of Flax: Linseed and Fibre Flax. Ind. Crops Prod. 2015, 68, 24–31. [Google Scholar] [CrossRef]
- Xu, K.; Li, X.Q.; Zhao, D.L.; Zhang, P. Antifungal secondary metabolites produced by the fungal endophytes: Chemical diversity and potential use in the development of biopesticides. Front. Microbiol. 2021, 12, 689527. [Google Scholar] [CrossRef]
- Toppo, P.; Kagatay, L.L.; Gurung, A.; Singla, P.; Chakraborty, R.; Roy, S.; Mathur, P. Endophytic fungi mediates production of bioactive secondary metabolites via modulation of genes involved in key metabolic pathways and their contribution in different biotechnological sector. 3 Biotech. 2023, 6, 191. [Google Scholar] [CrossRef]
- Baghbani, F.; Lotfi, R.; Moharramnejad, S.; Bandehagh, A.; Roostaei, M.; Rastogi, A.; Kalaji, H.M. Impact of Fusarium verticillioides on chlorophyll fluorescence parameters of two maize lines. Eur. J. Plant Pathol. 2019, 154, 337–346. [Google Scholar] [CrossRef]
- Alam, B.; Lǐ, J.; Gě, Q.; Khan, M.A.; Gōng, J.; Mehmood, S.; Yuán, Y.; Gǒng, W. Endophytic fungi: From symbiosis to secondary metabolite communications or vice versa? Front. Plant Sci. 2021, 12, 791033. [Google Scholar] [CrossRef] [PubMed]
- Davidova, S.; Galabov, A.S.; Satchanska, G. Antibacterial, Antifungal, Antiviral Activity, and Mechanisms of Action of Plant Polyphenols. Microorganisms 2024, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Aboody, M.S.A.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Antolín-Llovera, M.; Leivar, P.; Arró, M.; Ferrer, A.; Boronat, A.; Campos, N. Modulation of plant HMG-CoA reductase by protein phosphatase 2A: Positive and negative control at a key node of metabolism. Plant Signal Behav. 2011, 8, 1127–1131. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vibrat. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; J. Wiley & Sons: Chichester, UK, 2001; Chapter 2; p. 50. [Google Scholar]
- Wojtkowiak, B.; Chabanel, M. Spectrochimie Moleculaire; Technique et Documentation; PWN: Warszawa, Poland, 1984; Chapter 4; p. 114. [Google Scholar]
- Carrillo, F.; Colom, X.; Sunol, J.J.; Saurina, J. Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo, F. Crystallinity changes in lyocell and viscose-type fibres by caustic treatment. Eur. Polym. J. 2002, 38, 2225–2230. [Google Scholar] [CrossRef]
- Dai, D.; Fan, M. Investigation of the dislocation of natural fibres by Fourier-transform infrared spectroscopy. Vibrat. Spectrosc. 2011, 55, 300–306. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vibrat. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Kruer-Zerhusen, N.; Cantero-Tubilla, B.; Wilson, D.B. Characterization of cellulose crystallinity after enzymatic treatment using Fourier transform infrared spectroscopy (FTIR). Cellulose 2018, 25, 37–48. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; Van Dam, J.E.G. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Wojtasik, W.; Dymińska, L.; Hanuza, J.; Burgberger, M.; Boba, A.; Szopa, J.; Kulma, A.; Mierziak, J. Endophytic Non-Pathogenic Fusarium oxysporum Reorganizes the Cell Wall in Flax Seedlings. Front. Plant Sci. 2024, 15, 1352105. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Jeon, H.S.; Kim, S.H.; Chung, J.H.; Roppolo, D.; Lee, H.J.; Cho, H.J.; Tobimatsu, Y.; Ralph, J.; Park, O.K. Lignin-Based Barrier Restricts Pathogens to the Infection Site and Confers Resistance in Plants. EMBO J. 2019, 38, e101948. [Google Scholar] [CrossRef]
- Wróbel-Kwiatkowska, M.; Starzycki, M.; Zebrowski, J.; Oszmiański, J.; Szopa, J. Lignin Deficiency in Transgenic Flax Resulted in Plants with Improved Mechanical Properties. J. Biotechnol. 2007, 128, 919–934. [Google Scholar] [CrossRef]
- Miedes, E.; Vanholme, R.; Boerjan, W.; Molina, A. The Role of the Secondary Cell Wall in Plant Resistance to Pathogens. Front. Plant Sci. 2014, 5, 358. [Google Scholar] [CrossRef] [PubMed]
- Schuck, S.; Weinhold, A.; Luu, V.T.; Baldwin, I.T. Isolating fungal pathogens from a dynamic disease outbreak in a native plant population to establish plant-pathogen bioassays for the ecological model plant Nicotiana attenuata. PLoS ONE 2014, 9, e102915. [Google Scholar] [CrossRef]
- Wróbel-Kwiatkowska, M.; Słupczyńska, M.; Rymowicz, W. Overexpression of medium-chain-length polyhydroxyalkanoates induces significant salt tolerance and fungal resistance in flax. Plant Cell Tiss. Organ Cult. 2022, 151, 123–132. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown. J. Plant Physiol. 1987, 131, 101–110. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Blummel, M.; Becker, K. Formation of complexes between polyvinylpyrrolidones or polyethylene glycols and tannins, and their implication in gas production and true digestibility in in vitro techniques. Br. J. Nutr. 1995, 73, 897–913. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Naqvi, S.A.H.; Farhan, M.; Ahmad, M.; Kiran, R.; Shahbaz, M.; Abbas, A.; Hakim, F.; Shabbir, M.; Tan, Y.S.; Sathiya Seelan, J.S. Fungicide resistance in Fusarium species: Exploring environmental impacts and sustainable management strategies. Arch. Microbiol. 2025, 207, 31. [Google Scholar] [CrossRef] [PubMed]
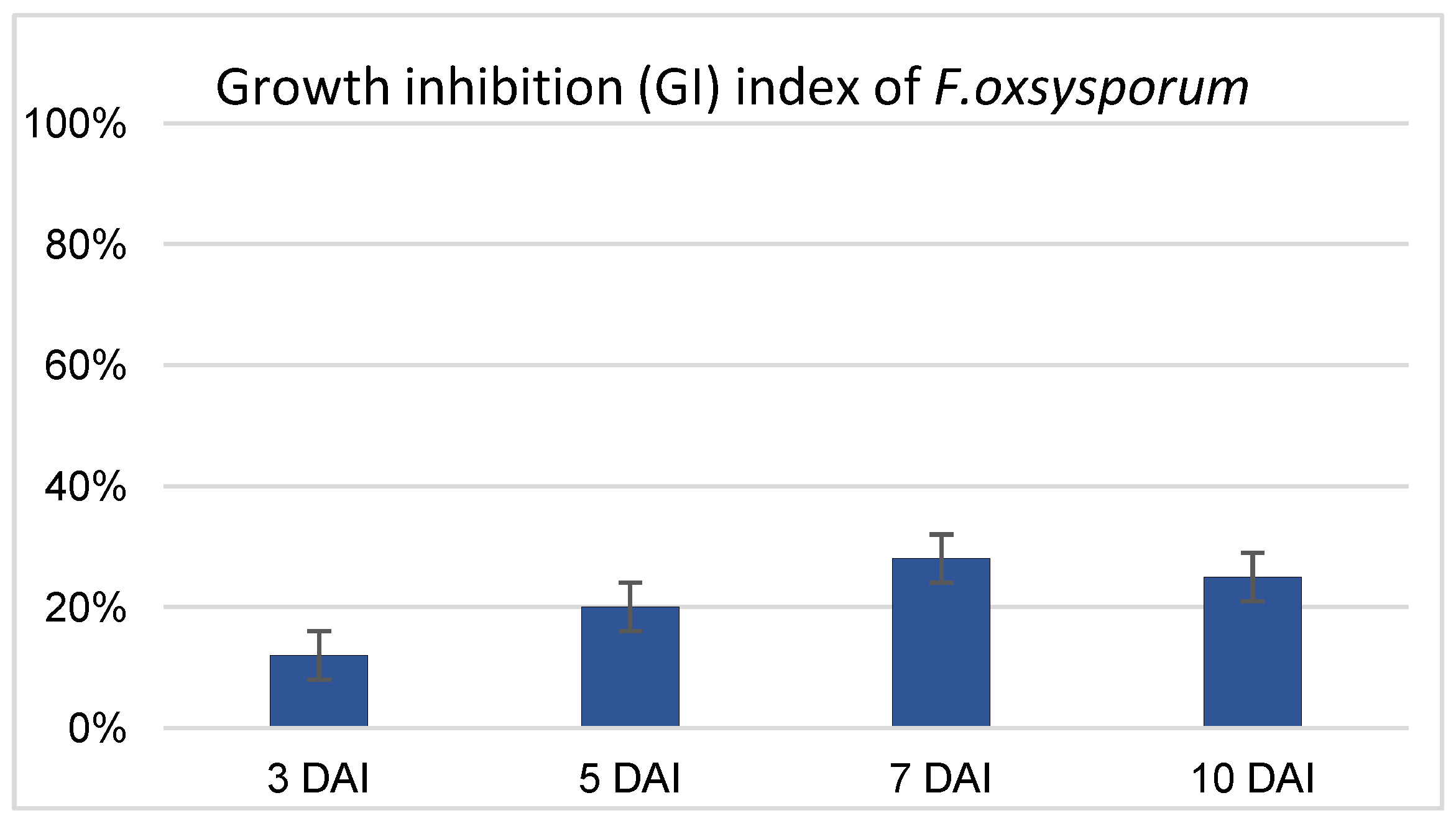
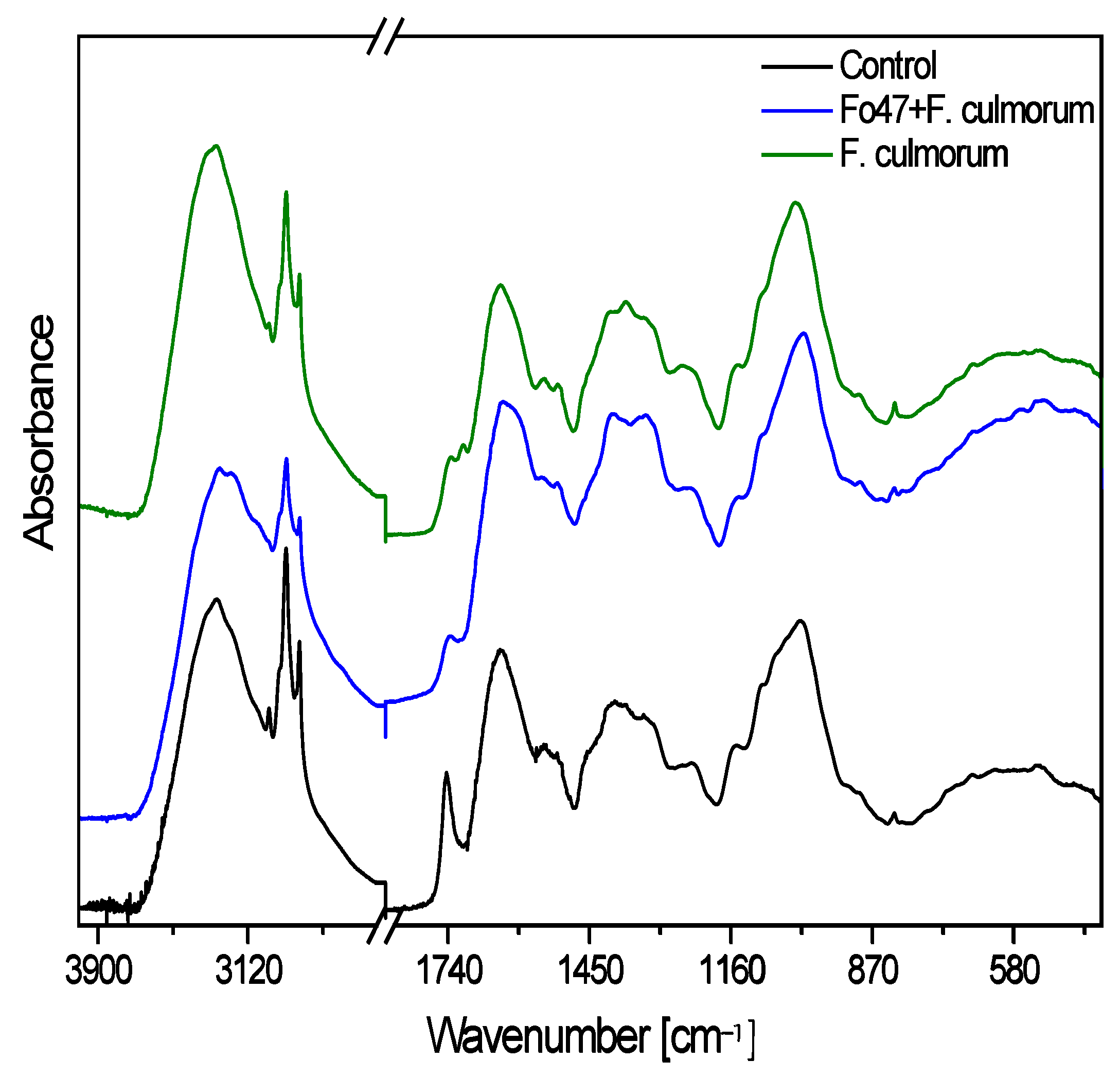
| Wavenumber (cm−1) | Assignments | Relationship |
|---|---|---|
| 1450 | δ(CH) + ω(CH2) + δ(OH) | Fc ≤ C < Fo47 + Fc |
| 1410 | δ(CH) + ν(φ) + δ(OH) | C ≤ Fo47 + Fc < Fc |
| 1315 | δ(CH) + δ(OH) | C ≤ Fc < Fo47 + Fc |
| 898 | ν(φ) + ρ(CH2) | C ≤ Fo47 + Fc ≤ Fc |
| 1150 | ν(C-O-C) | C ≈ Fo47 + Fc < Fc |
| Wavenumber (cm−1) | Assignments | Relationship |
|---|---|---|
| 1545 | ν(C=C) | C ≤ Fc < Fo47 + Fc |
| 1508 | ν(φ) | C ≤ Fc < Fo47 + Fc |
| 1336 | ν(C-O) + ν(φ) | C < Fc < Fo47 + Fc |
| 826 | δ(φ) + δ(CH) | C ≤ Fc < Fo47 + Fc |
| Wavenumber (cm−1) | Assignments | Relationship |
|---|---|---|
| 1740 | ester carbonyl groups ν(C=O) | C ≈ Fc < Fo47 + Fc |
| 1705 | ester carbonyl groups ν(C=O) | Fc ≤ C < Fo47 + Fc |
| 1647 | free or ionized carboxyl groups νas(COO−) | C ≤ Fc < Fo47 + Fc |
| 1600 | free or ionized carboxyl groups νs(COO−) | Fc ≤ C < Fo47 + Fc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liszka, J.; Dymińska, L.; Łaba, W.; Wróbel-Kwiatkowska, M. The Influence of the Non-Pathogenic Fusarium oxysporum Fo47 Strain on Flax Resistance to Pathogens. Int. J. Mol. Sci. 2025, 26, 4396. https://doi.org/10.3390/ijms26094396
Liszka J, Dymińska L, Łaba W, Wróbel-Kwiatkowska M. The Influence of the Non-Pathogenic Fusarium oxysporum Fo47 Strain on Flax Resistance to Pathogens. International Journal of Molecular Sciences. 2025; 26(9):4396. https://doi.org/10.3390/ijms26094396
Chicago/Turabian StyleLiszka, Justyna, Lucyna Dymińska, Wojciech Łaba, and Magdalena Wróbel-Kwiatkowska. 2025. "The Influence of the Non-Pathogenic Fusarium oxysporum Fo47 Strain on Flax Resistance to Pathogens" International Journal of Molecular Sciences 26, no. 9: 4396. https://doi.org/10.3390/ijms26094396
APA StyleLiszka, J., Dymińska, L., Łaba, W., & Wróbel-Kwiatkowska, M. (2025). The Influence of the Non-Pathogenic Fusarium oxysporum Fo47 Strain on Flax Resistance to Pathogens. International Journal of Molecular Sciences, 26(9), 4396. https://doi.org/10.3390/ijms26094396






