A Universal and Quantitative PCR Strategy for Detection and Epidemiologic Analysis of Canine Papillomavirus (CPV)
Abstract
1. Introduction
2. Results
2.1. Analyzing CPV L1 Sequences to Design PCR Primer Sets
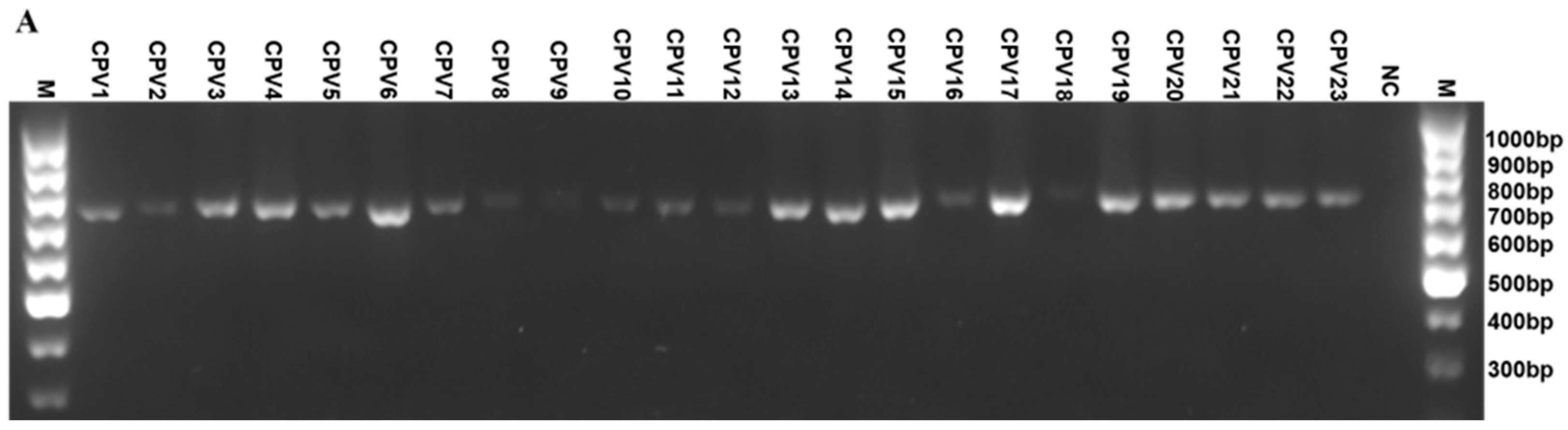
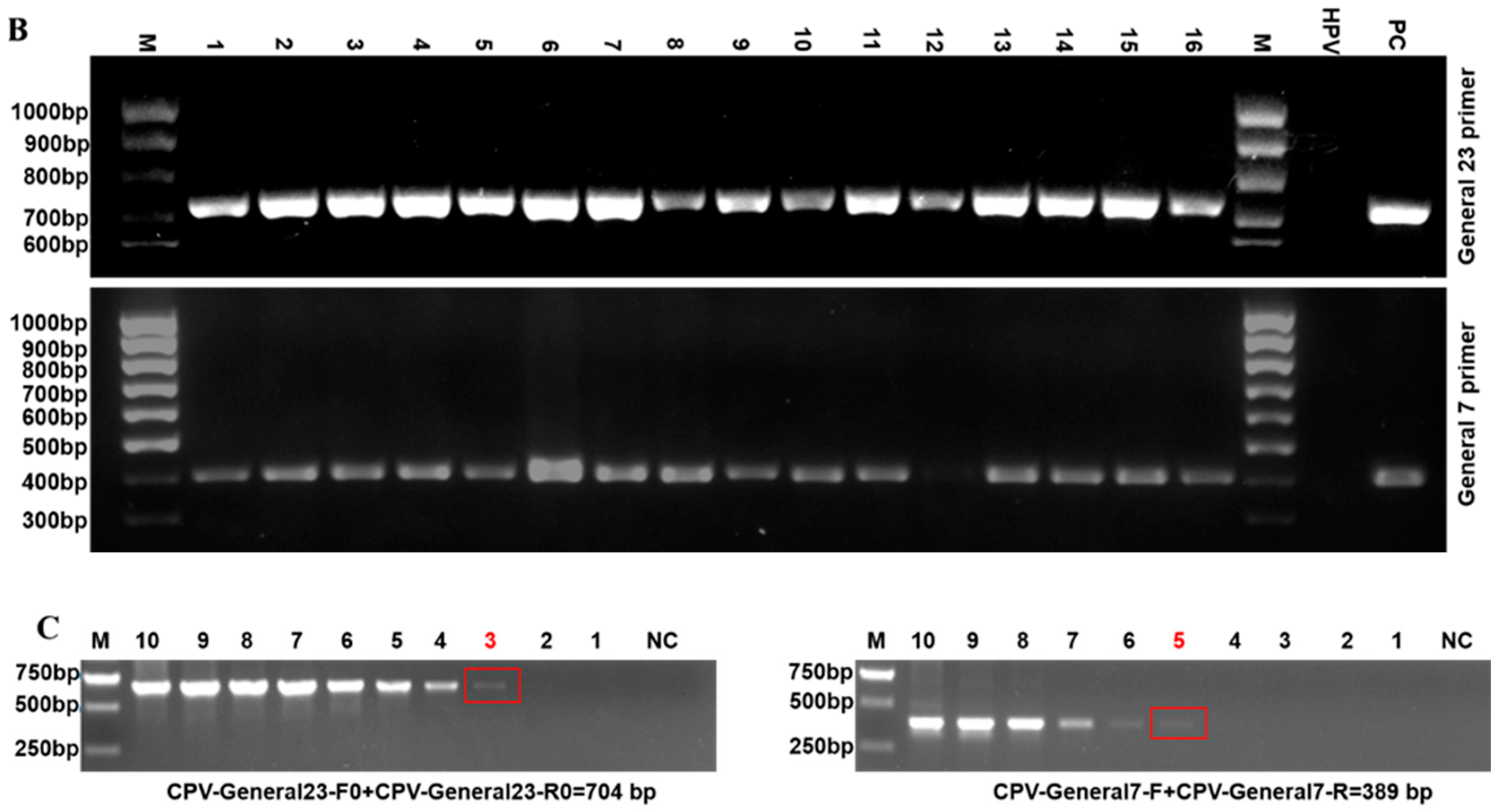
2.2. Establishing a Universal PCR Detection Assay for CPVs
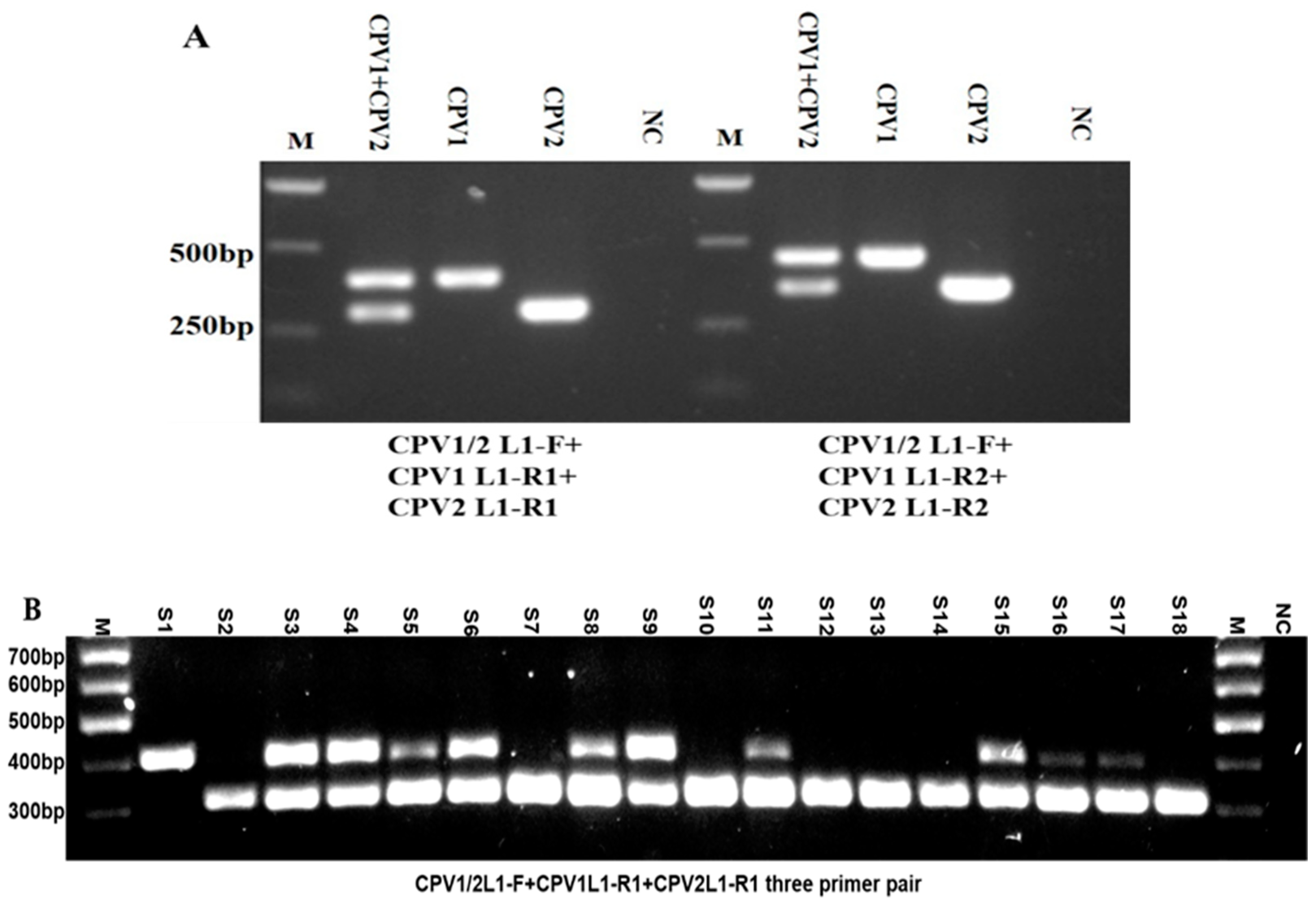
2.3. Developing a PCR Assay for Detection and Simultaneous Differentiation of CPV1 and CPV2
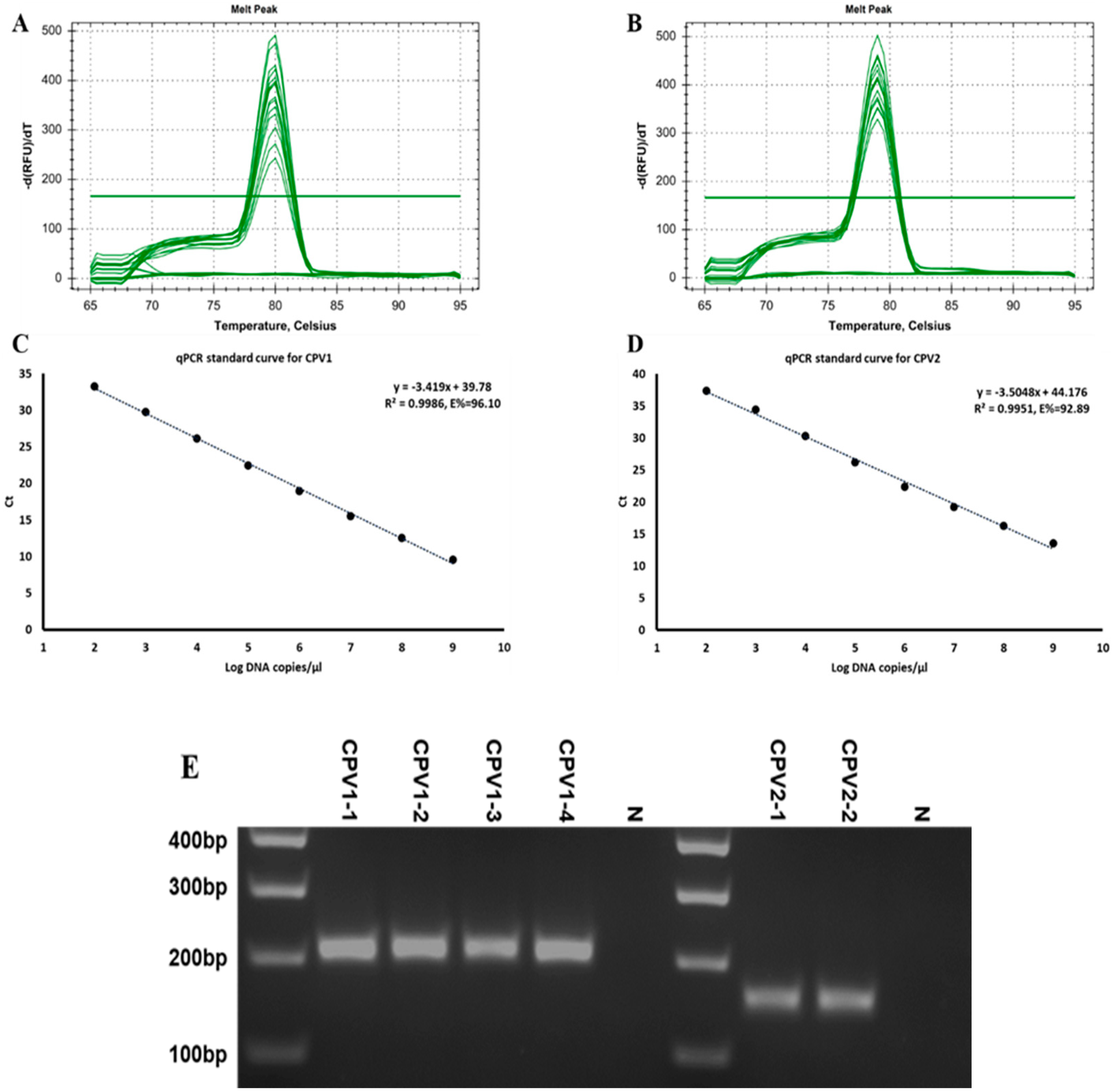
2.4. Implementing a Real-Time PCR Technique to Quantify the Viral Content of CPV1 and CPV2
2.5. Investigating the Epidemiology of CPVs
3. Materials and Methods
3.1. Sequence Analysis and Primer Design
3.2. Preparation of Standard or Positive Plasmids
3.3. Sample Collection and DNA Preparation
3.4. PCR and Real-Time Quantitative PCR (RT-qPCR)
3.5. Epidemiological Investigation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Pedraza-Chaverri, J. Pathological Similarities in the Development of Papillomavirus-Associated Cancer in Humans, Dogs, and Cats. Animals 2022, 12, 2390. [Google Scholar] [CrossRef]
- Medeiros-Fonseca, B.; Faustino-Rocha, A.I.; Medeiros, R.; Oliveira, P.A.; Gil da Costa, R.M. Canine and feline papillomaviruses: An update. Front. Vet. Sci. 2023, 10, 1174673. [Google Scholar] [CrossRef]
- Polinas, M.; Cacciotto, C.; Zobba, R.; Antuofermo, E.; Burrai, G.P.; Pirino, S.; Pittau, M.; Alberti, A. Ovine papillomaviruses: Diversity, pathogenicity, and evolution. Vet. Microbiol. 2024, 289, 109955. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, M.; Mazzei, M.; Vascellari, M.; Melchiotti, E.; Zanardello, C.; Verin, R.; Albanese, F.; Necci, F.; Pazzini, L.; Lazzarini, G.; et al. Localization and genotyping of canine papillomavirus in canine inverted papillomas. J. Vet. Diagn. Invest. 2021, 33, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.D.R.; Batista, M.V.A. New insights into Canis familiaris papillomaviruses genetics and biology: Is the genetic characterization of CPV types and their variants an important clinical issue? Genet. Mol. Biol. 2022, 45 (Suppl. S1), e20210388. [Google Scholar] [CrossRef]
- Munday, J.S.; Gedye, K.; Knox, M.A.; Robinson, L.; Lin, X. Genomic Characterization of Canis Familiaris Papillomavirus Type 25, a Novel Papillomavirus Associated with a Viral Plaque from the Pinna of a Dog. Animals 2023, 13, 1859. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Sundberg, J.P.; Goldschmidt, M.H.; Knupp, C.; Reichmann, M.E. Cutaneous inverted papillomas in dogs. Vet. Pathol. 1988, 25, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wang, A.; Maxwell, S.; Schlegel, R.; Yuan, H. Long-Term Culture of Canine Ocular Cells That Maintain Canine Papillomaviruses. Viruses 2022, 14, 2675. [Google Scholar] [CrossRef]
- Resendes, A.R.; Trainor, K.E.; Bera, M.; Cheng, R.C.F.; Luff, J. Claw bed inverted squamous papilloma associated with canine papillomavirus type 2 in a dog. Vet. Dermatol. 2024, 35, 230–233. [Google Scholar] [CrossRef]
- Yhee, J.Y.; Kwon, B.J.; Kim, J.H.; Yu, C.H.; Im, K.S.; Lee, S.S.; Lyoo, Y.S.; Chang, B.J.; Sur, J.H. Characterization of canine oral papillomavirus by histopathological and genetic analysis in Korea. J. Vet. Sci. 2010, 11, 21–25. [Google Scholar] [CrossRef]
- Yuan, H.; Ghim, S.; Newsome, J.; Apolinario, T.; Olcese, V.; Martin, M.; Delius, H.; Felsburg, P.; Jenson, B.; Schlegel, R. An epidermotropic canine papillomavirus with malignant potential contains an E5 gene and establishes a unique genus. Virology 2007, 359, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Plattner, B.L.; Hostetter, J.M. Cutaneous viral papilloma with local extension and subungual cyst formation in a dog. J. Vet. Diagn. Invest. 2009, 21, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, T.R.; Chamings, A.; Vibin, J.; Alexandersen, S. Detection and characterisation of canine astrovirus, canine parvovirus and canine papillomavirus in puppies using next generation sequencing. Sci. Rep. 2019, 9, 4602. [Google Scholar] [CrossRef]
- Lange, C.E.; Jennings, S.H.; Diallo, A.; Lyons, J. Canine papillomavirus types 1 and 2 in classical papillomas: High abundance, different morphological associations and frequent co-infections. Vet. J. 2019, 250, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, N.; Nespeca, G.; Hauser, B.; Ackermann, M.; Favrot, C. Detection of novel papillomaviruses in canine mucosal, cutaneous and in situ squamous cell carcinomas. Vet. Dermatol. 2005, 16, 290–298. [Google Scholar] [CrossRef]
- Yuan, H.; Estes, P.A.; Chen, Y.; Newsome, J.; Olcese, V.A.; Garcea, R.L.; Schlegel, R. Immunization with a pentameric L1 fusion protein protects against papillomavirus infection. J. Virol. 2001, 75, 7848–7853. [Google Scholar] [CrossRef]
- Munday, J.S.; Bond, S.D.; Piripi, S.; Soulsby, S.J.; Knox, M.A. Canis Familiaris Papillomavirus Type 26: A Novel Papillomavirus of Dogs and the First Canine Papillomavirus within the Omegapapillomavirus Genus. Viruses 2024, 16, 595. [Google Scholar] [CrossRef]
- Munday, J.S.; Gedye, K.; Knox, M.A.; Ravens, P.; Lin, X. Genomic Characterisation of Canis Familiaris Papillomavirus Type 24, a Novel Papillomavirus Associated with Extensive Pigmented Plaque Formation in a Pug Dog. Viruses 2022, 14, 2357. [Google Scholar] [CrossRef]
- Alves, C.; Weber, M.N.; Guimaraes, L.L.B.; Cibulski, S.P.; da Silva, F.R.C.; Daudt, C.; Budaszewski, R.F.; Silva, M.S.; Mayer, F.Q.; Bianchi, R.M.; et al. Canine papillomavirus type 16 associated to squamous cell carcinoma in a dog: Virological and pathological findings. Braz. J. Microbiol. 2020, 51, 2087–2094. [Google Scholar] [CrossRef]
- Luff, J.A.; Affolter, V.K.; Yeargan, B.; Moore, P.F. Detection of six novel papillomavirus sequences within canine pigmented plaques. J. Vet. Diagn. Invest. 2012, 24, 576–580. [Google Scholar] [CrossRef]
- Orlandi, M.; Mazzei, M.; Albanese, F.; Pazzini, L.; Mei, M.; Lazzarini, G.; Forzan, M.; Massaro, M.; Vascellari, M.; Abramo, F. Clinical, histopathological, and molecular characterization of canine pigmented viral plaques. Vet. Pathol. 2023, 60, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.E.; Tobler, K.; Schraner, E.M.; Vetsch, E.; Fischer, N.M.; Ackermann, M.; Favrot, C. Complete canine papillomavirus life cycle in pigmented lesions. Vet. Microbiol. 2013, 162, 388–395. [Google Scholar] [CrossRef]
- Chang, C.Y.; Yamashita-Kawanishi, N.; Tomizawa, S.; Liu, I.L.; Chen, W.T.; Chang, Y.C.; Huang, W.H.; Tsai, P.S.; Shirota, K.; Chambers, J.K.; et al. Whole Genomic Analysis and Comparison of Two Canine Papillomavirus Type 9 Strains in Malignant and Benign Skin Lesions. Viruses 2020, 12, 736. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Chen, W.T.; Haga, T.; Yamashita, N.; Lee, C.F.; Tsuzuki, M.; Chang, H.W. The Detection and Association of Canine Papillomavirus with Benign and Malignant Skin Lesions in Dogs. Viruses 2020, 12, 170. [Google Scholar] [CrossRef]
- Gil da Costa, R.M.; Peleteiro, M.C.; Pires, M.A.; DiMaio, D. An Update on Canine, Feline and Bovine Papillomaviruses. Transbound. Emerg. Dis. 2017, 64, 1371–1379. [Google Scholar] [CrossRef]
- Munday, J.S.; Orbell, G.; Robinson, L. Detection of a novel papillomaviral sequence in viral plaques confined to the pinna of a dog. Vet. Dermatol. 2023, 34, 367–370. [Google Scholar] [CrossRef]
- Lange, C.E.; Zollinger, S.; Tobler, K.; Ackermann, M.; Favrot, C. Clinically healthy skin of dogs is a potential reservoir for canine papillomaviruses. J. Clin. Microbiol. 2011, 49, 707–709. [Google Scholar] [CrossRef]
- Merchioratto, I.; Mucellini, C.I.; Lopes, T.R.R.; de Oliveira, P.S.B.; Silva Junior, J.V.J.; Brum, M.C.S.; Weiblen, R.; Flores, E.F. Phylogenetic analysis of papillomaviruses in dogs from southern Brazil: Molecular epidemiology and investigation of mixed infections and spillover events. Braz. J. Microbiol. 2024, 55, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chambers, J.K.; Tsuzuki, M.; Yamashita, N.; Ushigusa, T.; Haga, T.; Nakayama, H.; Uchida, K. Pigmented viral plaque and basal cell tumor associated with canine papillomavirus infection in Pug dogs. J. Vet. Med. Sci. 2019, 81, 1643–1648. [Google Scholar] [CrossRef]
- Ayala-Diaz, S.; Jimenez-Lima, R.; Ramirez-Alcantara, K.M.; Lizano, M.; Castro-Munoz, L.J.; Reyes-Hernandez, D.O.; Arroyo-Ledezma, J.; Manzo-Merino, J. Presence of Papillomavirus DNA sequences in the canine transmissible venereal tumor (CTVT). PeerJ 2019, 7, e7962. [Google Scholar] [CrossRef]
- Thaiwong, T.; Sledge, D.G.; Wise, A.G.; Olstad, K.; Maes, R.K.; Kiupel, M. Malignant transformation of canine oral papillomavirus (CPV1)-associated papillomas in dogs: An emerging concern? Papillomavirus Res. 2018, 6, 83–89. [Google Scholar] [CrossRef] [PubMed]
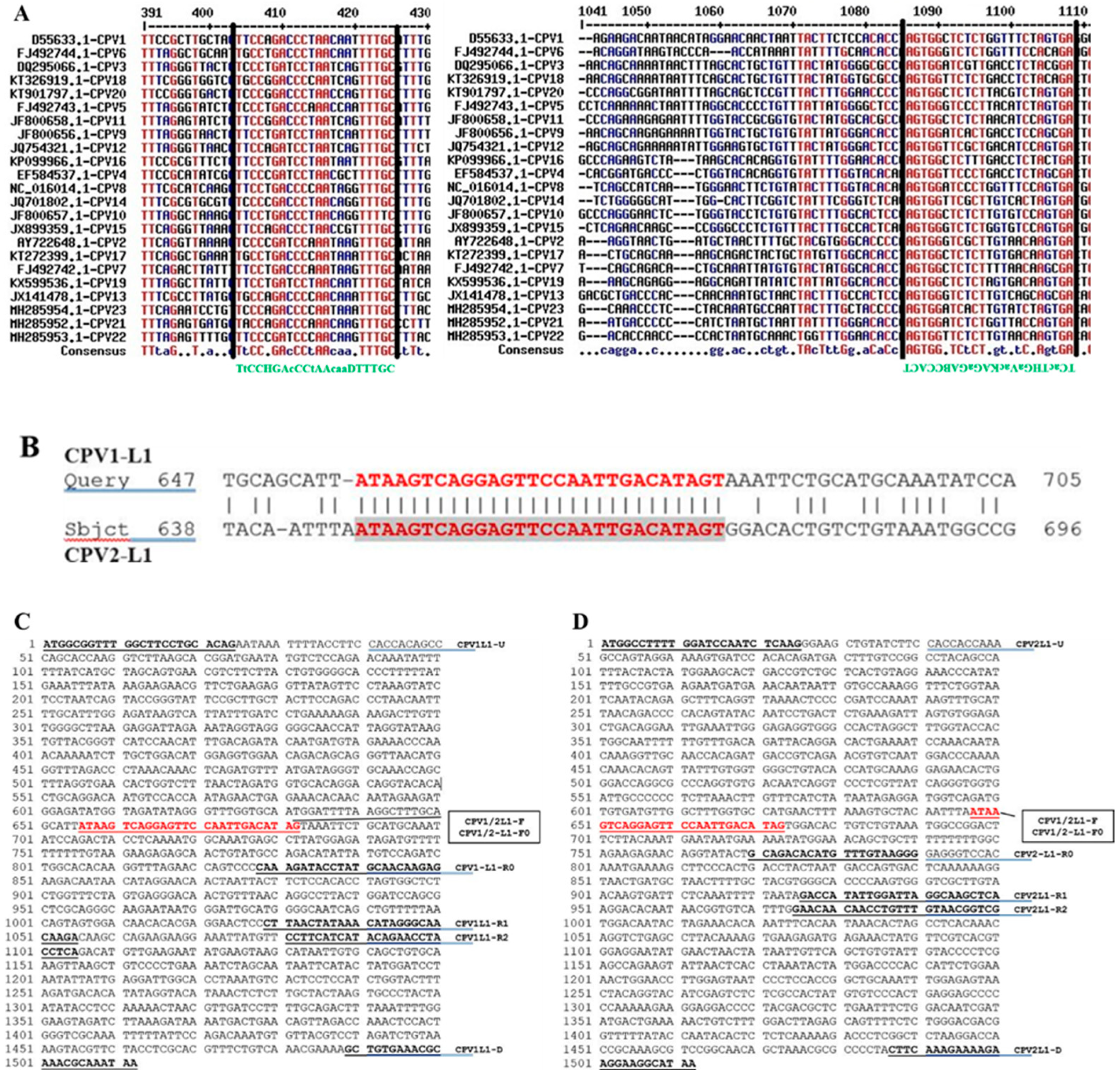
| Primer Category | Primers | Sequence (5′-3′) | Amplicon Size | Application |
|---|---|---|---|---|
| General detection primers | CPV-General23-F0 | TtCCHGAcCCtAAcaaDTTTGC | 673~704 bp | General detection CPV1-23 |
| CPV-General23-R0 | TCacTHGaVacKAGaGABCCACT | |||
| CPV1/CPV2 typing and identification primers (triple primer method) | CPV1/2L1-F | ATAAGTCAGGAGTTCCAATTGACATAG | 300/400 bp or 350/450 bp (depending on the downstream primers) | Simultaneously detect and distinguish between CPV1 and CPV2 infections |
| CPV1L1-R1 | TCTTGTTGCCCTATGTTTATAGTTAAG | 400 bp (in combination with CPV1/2L1-F) | ||
| CPV1L1-R2 | TGAGCTTGCCTAATCCAATATGGTC | 300 bp (in combination with CPV1/2L1-F) | ||
| CPV2L1-R1 | TGAGGTAGGTTCTGTATGATGAAGG | 450 bp (in combination with CPV1/2L1-F) | ||
| CPV2L1-R2 | CGACCGTTACAAACAGGTTGTTGTTC | 350 bp (in combination with CPV1/2L1-F) | ||
| Full-length L1 gene amplification primers | CPV1L1-U | ATGGCGGTTT GGCTTCCTGC ACAG | 1512 bp | Amplify full-length CPV1-L1 gene |
| CPV1L1-D | TTATTTGCGTTTGCGTTTCACAGC | |||
| CPV2L1-U | ATGGCCTTTT GGATCCAATCTCAAG | Amplify full-length CPV2-L1 gene | ||
| CPV2L1-D | TTATGCCTTCCTTCTTTTCTTTGAAG | |||
| Quantitative detection primers (for qPCR) | CPV1/2-L1-F0 | TCAGGAGTTCCAATTGACATAG | 140/190 bp (depending on downstream primers) | Specific quantification of CPV1 or CPV2 viral content |
| CPV1-L1-R0 | CTCTTGTTGCATAGGTATCTTTG | 190 bp (in combination with CPV1/2-L1-F0) | ||
| CPV2-L1-R0 | CCCTTACAAACATGTGTCTGC | 140 bp (in combination with CPV1/2-L1-F0) |
| Genotypes | Samples | Ct Value (Mean ± SD, n = 3) | Viral Content (Copies/µL) |
|---|---|---|---|
| CPV1 | 1 | 11.72 ± 0.14 | 1.1 × 108.21±0.04 |
| 2 | 17.97 ± 0.23 | 1.1 × 106.38±0.07 | |
| 3 | 10.43 ± 0.08 | 1.1 × 108.59±0.02 | |
| 4 | 12.50 ± 0.12 | 1.1 × 107.98±0.04 | |
| CPV2 | 1 | 13.87 ± 0.13 | 1.1 × 108.65±0.04 |
| 2 | 14.97 ± 0.05 | 1.1 × 108.33±0.01 |
| Year | CPV1 (n)/ Percentage (%) | CPV2 (n) /Percentage (%) | Other Genotypes (n) /Percentage (%) | Co-Infection by CPV1 and CPV2 (n)/Percentage (%) | Annual Total (n) |
|---|---|---|---|---|---|
| 2017 | 17 (80.95%) | 3 (14.29%) | 1 (4.76%) | 0 (0.00%) | 21 |
| 2018 | 32 (94.12%) | 0 (0.00%) | 1 (2.94%) | 1 (2.94%) | 34 |
| 2019 | 19 (63.33%) | 5 (16.67%) | 1 (3.33%) | 5 (16.67%) | 30 |
| 2020 | 18 (75.00%) | 2 (8.33%) | 2 (8.33%) | 2 (8.33%) | 24 |
| 2021 | 23 (85.19%) | 1 (3.70%) | 1 (3.70%) | 2 (7.40%) | 27 |
| Total (n)/ Percentage | 109 (80.15%) | 11 (8.09%) | 6 (4.41%) | 10 (7.35%) | 136 |
| Sample Source | Sample Type | CPV1 (n)/ Percentage (%) | CPV2 (n) /Percentage (%) | CPV1+CPV2 (n) /Percentage (%) | Total (n) |
|---|---|---|---|---|---|
| Animal hospital | Oral swab | 0 (0.00%) | 4 (19.48%) | 2 (9.52%) | 21 |
| Animal Rescue center | Oral swab | 32 (62.74%) | 14 (27.45%) | 5 (9.80%) | 51 |
| Professional dog breeding facility | Oral swab | 6 (33.33%) | 10 (55.56%) | 2 (11.11%) | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Wang, K.; Yuan, Y.; Li, Y.; Schlegel, R.; Wang, A.; Yuan, H. A Universal and Quantitative PCR Strategy for Detection and Epidemiologic Analysis of Canine Papillomavirus (CPV). Int. J. Mol. Sci. 2025, 26, 4391. https://doi.org/10.3390/ijms26094391
Zhou D, Wang K, Yuan Y, Li Y, Schlegel R, Wang A, Yuan H. A Universal and Quantitative PCR Strategy for Detection and Epidemiologic Analysis of Canine Papillomavirus (CPV). International Journal of Molecular Sciences. 2025; 26(9):4391. https://doi.org/10.3390/ijms26094391
Chicago/Turabian StyleZhou, Dan, Kaixin Wang, Youming Yuan, Yalan Li, Richard Schlegel, Aibing Wang, and Hang Yuan. 2025. "A Universal and Quantitative PCR Strategy for Detection and Epidemiologic Analysis of Canine Papillomavirus (CPV)" International Journal of Molecular Sciences 26, no. 9: 4391. https://doi.org/10.3390/ijms26094391
APA StyleZhou, D., Wang, K., Yuan, Y., Li, Y., Schlegel, R., Wang, A., & Yuan, H. (2025). A Universal and Quantitative PCR Strategy for Detection and Epidemiologic Analysis of Canine Papillomavirus (CPV). International Journal of Molecular Sciences, 26(9), 4391. https://doi.org/10.3390/ijms26094391






